Evidence for a protein tether involved in somatic touch - PubMed (original) (raw)
Evidence for a protein tether involved in somatic touch
Jing Hu et al. EMBO J. 2010.
Abstract
The gating of ion channels by mechanical force underlies the sense of touch and pain. The mode of gating of mechanosensitive ion channels in vertebrate touch receptors is unknown. Here we show that the presence of a protein link is necessary for the gating of mechanosensitive currents in all low-threshold mechanoreceptors and some nociceptors of the dorsal root ganglia (DRG). Using TEM, we demonstrate that a protein filament with of length approximately 100 nm is synthesized by sensory neurons and may link mechanosensitive ion channels in sensory neurons to the extracellular matrix. Brief treatment of sensory neurons with non-specific and site-specific endopeptidases destroys the protein tether and abolishes mechanosensitive currents in sensory neurons without affecting electrical excitability. Protease-sensitive tethers are also required for touch-receptor function in vivo. Thus, unlike the majority of nociceptors, cutaneous mechanoreceptors require a distinct protein tether to transduce mechanical stimuli.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
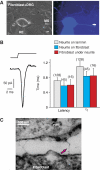
Fibroblast/sensory neuron co-cultures suggest a tether gating mechanism. (A) Bright-field (left) and fluorescence micrograph (right) of a recorded neuron on fibroblasts, filled with Lucifer Yellow from the recording pipette (RE). Mechanical stimuli (MS, amplitude 750 nm) were applied either to the neurite directly or to the adjacent fibroblast (indicated by white arrow). (B) Example of an RA-mechanosensitive current in a sensory neuron evoked by a 750-nm displacement of a fibroblast adjacent to the neurite. The bar graph shows the mean latency and mean activation time constant τ1 (obtained from mono-exponential fits of the current trace) for the mechanosensitive current evoked from the neurite on laminin (grey), neurite on fibroblast (blue) and from the fibroblast underneath the neurite (red). (C) Electron micrograph of electron-dense filaments observed between neurites and fibroblasts in a sensory neurons/fibroblast co-culture. Error bars represent the s.e.m.
Figure 2
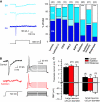
Subtilisin and blisterase selectively abolish RA-mechanosensitive currents. (A) Examples of RA-, IA- and SA-mechanosensitive currents evoked by stimulating sensory neuron neurites. Stacked histograms of the proportion of the three types of mechanosensitive current observed in controls (laminin and laminin-111) as compared with those in cultures treated with agents that disrupt extracellular matrix–cell interactions. The number of recorded neurons is indicated at the top of each stacked histogram. The empty bars indicate neurons in which no mechanosensitive current could be measured. (B) Local perfusion of single neuron with subtilisin leads to complete loss of the RA-mechanosensitive current (left); the current injection is, however, still effective at initiating APs. (C) The resting membrane potential (RMP), and the threshold current for AP initiation, was measured 0–3 h after subtilisin (red bars) or blisterase treatment (grey bars); the data were analysed separately for large (>25 μm diameter) and small neurons (<25 μm diameter); no differences were noted as compared with controls (black bars). Error bars represent ±s.e.m.
Figure 3
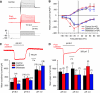
Protease treatment does not affect other ion channels. (A) Example traces of whole-cell currents evoked by a series of step depolarization steps from a −120-mV pre-pulse potential in 10-mV steps, to +50 mV. The black traces are from the neurons before subtilisin treatment and the red traces were obtained post-subtilisin; no major change was seen in the kinetics or amplitudes of inward and outward currents. (B) Mean whole-cell inward and outward currents measured at different test potentials for all control cells (black) and cells treated acutely with subtilisin (red) or blisterase (blue). At each test potential, the peak inward current peak outward current at steady state was measured. Neurons treated with subtilisin showed small, but significant, leftward shift in the voltage for peak activation of the inward current (asterisks indicate data points significantly different from the control; two-way ANOVA, Bonferroni post hoc test _P_>0.01). No change was observed in blisterase-treated cells as compared with that in the controls. (C, D) Proton-gated currents are not altered after subtilisin and blisterase treatment. The peak sustained (example trace in panel C) and transient (example trace in panel D) were not altered after subtilisin treatment. The amplitudes of proton-gated currents measured with stimuli of pH 6.0, 5.0 and 4.0 were not significantly different between control (black), subtilisin-treated (red) and blisterase-treated (blue) cells. The numbers of cells measured in each group is indicated in parentheses above each column.
Figure 4
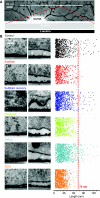
TEM reveals a protein filament necessary for mechanotransduction. (A) Light photomicrograph of a cultured sensory neuron with a neuritic tree. The TEM photomicrographs were generated from ultrathin sections from blocks of tissue, with dimensions in the range illustrated by the white rectangle at the end of red dotted lines. (B) Sample electron micrographs of the neurite–matrix interface under a series of conditions, including, in each case, a quantification of the length of each measured attachment plotted in random two-dimensional space to illustrate the range of attachment lengths observed (right). Objects larger and smaller than 75 nm are demarcated by the red line. For each experimental condition, two sample photomicrographs are shown: one field illustrating tight attachment of the plasma membrane to the substrate and one a looser attachment area, with or without long protein tethers. For each experimental condition, a colour code is applied as follows: from the top, control (black), subtilisin acute (red), subtilisin recovery (purple), blisterase treatment acute (green), PIPLC treatment acute (cyan) and SCG neurons on laminin (orange).
Figure 5
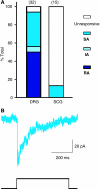
Superior sympathetic neurons lack an RA-mechanosensitive current (A). A stacked histogram of the proportion of three types of mechanically activated currents designated RA (dark blue), SA (medium blue) or IA (light blue) observed in DRG neurons and SCG neurons. (B) Example of an SA-mechanosensitive current recorded in an SCG neuron.
Figure 6
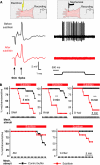
Mechanoreceptors, but not nociceptors, require a subtilisin-sensitive protein. (A) A schematic representation of the recording setup using the in vitro skin-nerve preparation and an example of a recording from a mechanoreceptor (SAM) before (black trace) and after application of subtilisin locally to the receptive field (red trace). Note that the electrically evoked spike is unaffected by the treatment, but the fibre's mechanosensitivity was completely abolished. (B) Kaplan–Meyer plots of the percentage of sensory fibres with a mechanosensitivity before and after local application of subtilisin. The top row contains data from three types of mechanoreceptor (RAM, SAM and D-hair are shown; note that subtilisin abolishes mechanosensitivity in all three receptors, with a median survival time as indicated). The bottom row shows the same experiment for A-fibre nociceptors (AMs) and for nociceptive C-fibres; no significant effect of subtilisin was observed. *** indicates statistical significance (P<0.005 log-rank test).
Figure 7
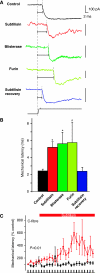
Protease modulation of transduction speed. (A) The latency of the SA-mechanosensitive current was measured in controls and protease-treated cells. Note that all protease treatments led to clear increase in the mechanical latency of the current (horizontal lines) as compared with that in the control (black trace), and this recovered to control values after recovery (blue trace bottom). Note that all vertical scale bars denote 100 pA. (B) Quantification of data shown in panel A; all three protease treatments lead to increased latency of the SA-mechanosensitive current. (C) The mechanical latency of C-fibre nociceptors treated at the receptive field with subtilisin is significantly increased following the application in the skin-nerve preparation. Statistical significance was calculated by repeated-measures ANOVA.
Similar articles
- Piezo2 voltage-block regulates mechanical pain sensitivity.
Sánchez-Carranza O, Chakrabarti S, Kühnemund J, Schwaller F, Bégay V, García-Contreras JA, Wang L, Lewin GR. Sánchez-Carranza O, et al. Brain. 2024 Oct 3;147(10):3487-3500. doi: 10.1093/brain/awae227. Brain. 2024. PMID: 38984717 Free PMC article. - Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons.
Hao J, Delmas P. Hao J, et al. J Neurosci. 2010 Oct 6;30(40):13384-95. doi: 10.1523/JNEUROSCI.2926-10.2010. J Neurosci. 2010. PMID: 20926665 Free PMC article. - Piezo2 is the major transducer of mechanical forces for touch sensation in mice.
Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Ranade SS, et al. Nature. 2014 Dec 4;516(7529):121-5. doi: 10.1038/nature13980. Nature. 2014. PMID: 25471886 Free PMC article. - Transduction and encoding sensory information by skin mechanoreceptors.
Hao J, Bonnet C, Amsalem M, Ruel J, Delmas P. Hao J, et al. Pflugers Arch. 2015 Jan;467(1):109-19. doi: 10.1007/s00424-014-1651-7. Epub 2014 Nov 23. Pflugers Arch. 2015. PMID: 25416542 Review. - Peripheral Mechanobiology of Touch-Studies on Vertebrate Cutaneous Sensory Corpuscles.
Cobo R, García-Piqueras J, García-Mesa Y, Feito J, García-Suárez O, Vega JA. Cobo R, et al. Int J Mol Sci. 2020 Aug 27;21(17):6221. doi: 10.3390/ijms21176221. Int J Mol Sci. 2020. PMID: 32867400 Free PMC article. Review.
Cited by
- Touch sense: functional organization and molecular determinants of mechanosensitive receptors.
Roudaut Y, Lonigro A, Coste B, Hao J, Delmas P, Crest M. Roudaut Y, et al. Channels (Austin). 2012 Jul-Aug;6(4):234-45. doi: 10.4161/chan.22213. Channels (Austin). 2012. PMID: 23146937 Free PMC article. Review. - Role of microtubules in Piezo2 mechanotransduction of mouse Merkel cells.
Chang W, Gu JG. Chang W, et al. J Neurophysiol. 2020 Dec 1;124(6):1824-1831. doi: 10.1152/jn.00502.2020. Epub 2020 Oct 21. J Neurophysiol. 2020. PMID: 33085566 Free PMC article. - Somatosensory neurons integrate the geometry of skin deformation and mechanotransduction channels to shape touch sensing.
Sanzeni A, Katta S, Petzold B, Pruitt BL, Goodman MB, Vergassola M. Sanzeni A, et al. Elife. 2019 Aug 13;8:e43226. doi: 10.7554/eLife.43226. Elife. 2019. PMID: 31407662 Free PMC article. - The cell biology of touch.
Lumpkin EA, Marshall KL, Nelson AM. Lumpkin EA, et al. J Cell Biol. 2010 Oct 18;191(2):237-48. doi: 10.1083/jcb.201006074. J Cell Biol. 2010. PMID: 20956378 Free PMC article. Review. - Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch.
Poole K, Herget R, Lapatsina L, Ngo HD, Lewin GR. Poole K, et al. Nat Commun. 2014 Mar 24;5:3520. doi: 10.1038/ncomms4520. Nat Commun. 2014. PMID: 24662763 Free PMC article.
References
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB (2006) The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci 26: 7022–7034 - PMC - PubMed
- Assad JA, Shepherd GM, Corey DP (1991) Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7: 985–994 - PubMed
- Bravo DA, Gleason JB, Sanchez RI, Roth RA, Fuller RS (1994) Accurate and efficient cleavage of the human insulin proreceptor by the human proprotein-processing protease furin. Characterization and kinetic parameters using the purified, secreted soluble protease expressed by a recombinant baculovirus. J Biol Chem 269: 25830–25837 - PubMed
- Chalfie M (2009) Neurosensory mechanotransduction. Nat Rev Mol Cell Biol 10: 44–52 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources