Transient elevation of adult hippocampal neurogenesis after dopamine depletion - PubMed (original) (raw)
Transient elevation of adult hippocampal neurogenesis after dopamine depletion
June-Hee Park et al. Exp Neurol. 2010 Apr.
Abstract
Degeneration of the midbrain dopaminergic neurons during Parkinson's disease (PD) may affect remote regions of the brain that are innervated by the projections of these neurons. The dentate gyrus (DG), a site of continuous production of new neurons in the adult hippocampus, receives dopaminergic inputs from the neurons of the substantia nigra (SN). Thus, depletion of the SN neurons during disease or in experimental settings may directly affect adult hippocampal neurogenesis. We show that experimental ablation of dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydopyridine (MPTP) mouse model of PD results in a transient increase in cell division in the subgranular zone (SGZ) of the DG. This increase is evident for the amplifying neural progenitors and for their postmitotic progeny; our results also indicate that MPTP treatment affects division of the normally quiescent stem cells in the SGZ. We also show that l-DOPA, used in the clinical treatment of PD, while attenuating the MPTP-induced death of dopaminergic neurons, does not alter the effect of MPTP on cell division in the DG. Our results suggest that a decrease in dopaminergic signaling in the hippocampus leads to a transient activation of stem and progenitor cells in the DG.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
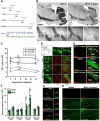
Figure 1. Treatment with MPTP induces transient increase in dividing cells in the DG
(A) Scheme of the experiment. Adult male mice were treated with MPTP or saline on day 1 and BrdU was injected 24 hr before each euthanasia point on the 4th, 7th, 14th, and 30th day (n=5 for each time point). (B) Single tile images of sagittal brain sections immunostained for tyrosine hydroxylase (TH). MPTP treatment destroys dopaminergic neurons in the midbrain and their axon fibers in the striatum and nucleus accumbens (NAc). Top row - saline- or MPTP-treated animals 7 days after the treatment; bottom row — higher magnification of the midbrain region 4, 7, or 14 days after the MPTP treatment or 14 days after saline treatment (control). Note the gradual disappearance of TH-stained dopaminergic neurons in the VTA and SN and loss of TH-positive fibers in the striatum and NAc. (C) Quantification of TH-immunoreactive (IR) cells in the SNpc and VTA. MPTP treatment results in a significant loss of TH IR neurons in the SNpc and VTA on the 7th and 14th day after the treatment compared to saline-treated controls (n=5 for each time point). Error bars show s.e.m. *_p_≤0.05 in Student's unpaired _t_-test. (D) Fluorescent Immunostaining for TH and Iba-1, a marker of microglia, 4 days after the MPTP or saline treatment. MPTP induces loss of TH-positive dopaminergic neurons in SN and VTA (top row). Hypertrophic Iba-1-stained microglia is seen near the VTA (third row) and the SNpc (bottom row). (E) Fluorescent immunostaining for BrdU, Iba-1, and TH 4 days after the MPTP or saline treatment in the SNpc, middle, and around the VTA, bottom. Most of the BrdU-labeled newborn cells in the SNpc and around the VTA are co-labeled with Iba-1. (F) Quantification of BrdU- and PCNA-IR cells in the DG 4, 7, 14, and 30 days after the MPTP treatment, with BrdU injected 24 hrs before the analysis. The results for individual animals are shown as black dots; error bas show s.e.m. *_p_≤0.05 in Student's unpaired _t_-test. (G) Fluorescent immunostaining for BrdU and PCNA in the DG 4 and 14 days after treatment with MPTP or saline. (H) Fluorescent immunostaining for BrdU and BLBP in the DG 14 days after treatment with MPTP or saline. The majority of newborn cells are in the SGZ, close to radial-glia like BLBP-positive QNP stem cells, below the granular cells layer. Some labeled cells can be seen in the hilus and molecular layer of the MPTP-treated animal brain. Abbreviations; cp, cerebral peduncle; Hip, hippocampus; LV, lateral ventricle; ml, medial lemniscus; MT, medial terminal nucleus of the accessory optic tract; SGZ, subgranular zone; SVZ, subventricular zone; SNpc, substantia nigra pars compacta; SNpr, substantia nigra pars reticulata; Tu, olfactory tubercle; VTA, ventral tegmental area; NAc; nucleus accumbens. Scale bars: 1 mm in B; 100 μm (top row) and 10 μm (middle and bottom rows) in D; 100 μm in E; 10 μm in G; 100 μm in H.
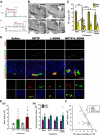
Figure 2. L-DOPA effects on cell death in the midbrain and cell division in the DG
(A) Scheme of the experiment. Adult male mice were treated with MPTP or saline on day 1 and L-DOPA and BZ or saline (control) were injected daily for 12 days, starting 1 or 14 days after the last MPTP injection. BrdU was injected 24 hr before euthanasia. (B) Single tile images of sagittal brain sections immunostained for tyrosine hydroxylase (TH). The loss of dopaminergic neurons and fibers is evident in the midbrain, striatum, and NAc and of their axon fibers in the striatum and nucleus accumbens (NAc). Top row - saline- or MPTP-treated animals; bottom row – L-DOPA or MPTP+L-DOPA 14 days after the treatment. Note that L-DOPA attenuates the MPTP-induced loss of TH-positive dopaminergic neurons in the SNpc and VTA and of their fibers in the striatum and NAc. (C) Quantification of TH-IR cells in the SNpc and VTA 14 days after the treatment with saline (n=4), MPTP (n=9), L-DOPA (n=5), or MPTP+L-DOPA (n=9). The results for individual animals are shown as black dots; error bas show s.e.m. *_p_≤0.05, **_p_≤0.01, ***_p_≤0.001 compared to the saline-treated group in ANOVA followed by Newman-Keuls post hoc test. (D) Fluorescent Immunostaining of cells in the DG for BrdU, PCNA and Prox-1. Most of the BrdU- and PCNA-labeled cells are distributed in the SGZ (top two rows, lower magnification), below the Prox-1-labeled granular cells of the DG (middle and bottom rows, showing granular cells and SGZ cells at higher magnification). (E) Quantification of BrdU-labeled cells in the DG 14 days (a) or 30 days (b) (see scheme in Fig, 3A) after the treatment with saline (n=4 for 14 days and 5 for 30 days), MPTP (n=9 for 14 days and 4 for 30 days), L-DOPA (n=5 for 14 days and 4 for 30 days), or MPTP+L-DOPA (n=9 for 14 days and 4 for 30 days), with BrdU injected 24 hrs before the analysis. The results for individual animals are shown as black dots; error bas show s.e.m. The variance of the values was significantly higher for the MPTP-exposed groups than for the saline- or L-DOPA-treated groups (p<0.0002 in the Bartlett's test for the 14 day set), the distribution of values was not Gaussian, and the number of subjects in each groups was different, indicating the use of a non-parametric Kruskal-Wallis test (_p_=0.0318) with the Dunn multiple comparison post hoc test; *p<0.05. (F) The loss of TH-positive dopaminergic neurons in the SNpc and cell division in the DG show a significant linear relationship in the MPTP+L-DOPA group 14 days after the treatment (regression coefficient, _r_2=0.4447; _p_=0.0498). Scale bars, 1 mm in B; 100 μm in the top two rows (BrdU/PCNA) in D and 10 μm in the middle row (BrdU/PCNA/Prox-1) in D.
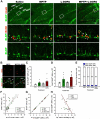
Figure 3. MPTP and L-DOPA affect progenitor cells in the DG
(A) Fluorescent Immunostaining of cells in the DG for BrdU and BLBP. Bracketed areas in the top row are presented at higher magnification in the bottom rows. QNPs are seen as BrdU+BLBP+ radial glia-like cells with a long basal process (white arrows), ANPs as BrdU+BLBP+ and BrdU+BLBP− compact cells with the basal process (white arrowheads), and OPCs as BrdU+BLBP+ and BrdU+BLBP− multiprocess cells (yellow arrowheads). Scale bars are 100 μm in the top row and 10 μm in the middle and bottom rows. (B) MPTP and MPTP+L-DOPA treatments increased cell proliferation in the molecular layer of the DG and stratum lacunosum-molecular of the hippocampus; however, most of the dividing cells were negative for BLBP immunostaining. Scale bar is 10 μm. (C) Quantification of BrdU+BLBP+ QNP cells in the DG 14 days after the treatment with saline (n=4), MPTP (n=9), L-DOPA (n=5), or MPTP+L-DOPA (n=9). The results for individual animals are shown as black dots; error bas show s.e.m. The variance of the values was significantly higher for the MPTP-exposed groups than for the saline- or L-DOPA-treated groups (p<0.0005 in the Bartlett's test), the distribution of values was not Gaussian, and the number of subjects in each groups was different, indicating the use of a non-parametric Kruskal-Wallis test with the Dunn multiple comparison post hoc test. (D) Quantification of BrdU+BLBP+ and BrdU+BLBP− ANP cells in the DG 14 days after the treatment with saline (n=4), MPTP (n=9), L-DOPA (n=5), or MPTP+L-DOPA (n=9). The results for individual animals are shown as black dots; error bas show s.e.m. The variance of the values was significantly higher for the MPTP-exposed groups than for the saline- or L-DOPA-treated groups (p<0.005 in the Bartlett's test), the distribution of values was not Gaussian, and the number of subjects in each groups was different, indicating the use of a non-parametric Kruskal-Wallis test (_p_=0.0307) with the Dunn multiple comparison post hoc test; _p_ = 0.052 for MPTP and 0.058 for MPTP+L-DOPA. (E) The fraction of BrdU-labeled QNP and ANPs cells among all BrdU-labeled cells in the SGZ. MPTP or L-DOPA treatments did not significantly change the fraction of BrdU-labeled QNP or ANPs cells (*_p_>0.05; Kruskal-Wallis test with the Dunn multiple comparison post hoc test). (F) The number of BrdU+/BLBP+ QNP cells shows a significant linear relationship (_r_2=0.4615 _p_=0.0442) with the number of BrdU+-labeled cells in the MPTP+L-DOPA group. (G) The number of BrdU+- labeled ANP cells shows a significant linear relationship (saline, r_2_=0.9988, _p_=0.0006; MPTP, _r_2=0.9989, _p_=0.0001; L-DOPA, r2=0.9761, _p_=0.0016; MPTP+LDOAP, _r_2=0.9973, _p_=0.0001) with the number of BrdU+-labeled cells in all animal groups. (H) The loss of TH-positive dopaminergic neurons in the SNpc and cell division in the DG show a significant linear relationship in the MPTP+L-DOPA group (_r_2=0.4625; _p_=0.0438).
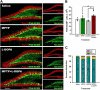
Figure 4. MPTP affects maturation of postmitotic neuronal progenitors in the DG
(A) Fluorescent immunostaining for PSA-NCAM and Prox-1, markers of potsmitioc neuroblasts and young neurons. Note that a fraction of PSA-NCAM- and Prox-1-expressing cells are localized in the middle and outer cell layer of the DG in the MPTP- and MPTP+L-DOPA-treated animals. Scale bar is 100 μm. (B) Quantification of PSA-NCAM-expressing cells in the DG 14 days after the treatment with saline (n=4), MPTP (n=9), L-DOPA (n=5), or MPTP+L-DOPA (n=9). The results for individual animals are shown as black dots; error bas show s.e.m. MPTP+L-DOPA increased the number of PSA-NCAM-labeled immature neurons in the DG, compared to the MPTP- and L-DOPA treated groups (_p_=0.0154 in Kruskal-Wallis test with the Dunn multiple comparison post hoc test; *p<0.05). (C) Distribution of PSA-NCAM expressing cells in different layers of the DG: the subgranular (SGZ), inner (IGZ), middle (MGZ), and outer (OGZ) granular zones of the DG. MPTP and MPTP+L-DOPA treatments increased the fraction of PSA-NCAM-expressing cells in the MGZ and the OGZ of the DG (_p_=0.0181, 0.0487, and 0.0114 in the Kruskal-Wallis test for the SGZ+IGZ, MGZ, and OGZ, respectively).
Similar articles
- Dopaminergic lesioning impairs adult hippocampal neurogenesis by distinct modification of α-synuclein.
Schlachetzki JC, Grimm T, Schlachetzki Z, Ben Abdallah NM, Ettle B, Vöhringer P, Ferger B, Winner B, Nuber S, Winkler J. Schlachetzki JC, et al. J Neurosci Res. 2016 Jan;94(1):62-73. doi: 10.1002/jnr.23677. Epub 2015 Oct 9. J Neurosci Res. 2016. PMID: 26451750 - Enhanced neuroinflammation and oxidative stress are associated with altered hippocampal neurogenesis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treated mice.
Singh S, Mishra A, Tiwari V, Shukla S. Singh S, et al. Behav Pharmacol. 2019 Dec;30(8):689-699. doi: 10.1097/FBP.0000000000000516. Behav Pharmacol. 2019. PMID: 31703031 - Indomethacin promotes survival of new neurons in the adult murine hippocampus accompanied by anti-inflammatory effects following MPTP-induced dopamine depletion.
Hain EG, Sparenberg M, Rasińska J, Klein C, Akyüz L, Steiner B. Hain EG, et al. J Neuroinflammation. 2018 May 26;15(1):162. doi: 10.1186/s12974-018-1179-4. J Neuroinflammation. 2018. PMID: 29803225 Free PMC article. - Physical activity and environmental enrichment regulate the generation of neural precursors in the adult mouse substantia nigra in a dopamine-dependent manner.
Klaissle P, Lesemann A, Huehnchen P, Hermann A, Storch A, Steiner B. Klaissle P, et al. BMC Neurosci. 2012 Oct 31;13:132. doi: 10.1186/1471-2202-13-132. BMC Neurosci. 2012. PMID: 23110504 Free PMC article. - Adult neurogenesis in Parkinson's disease.
Marxreiter F, Regensburger M, Winkler J. Marxreiter F, et al. Cell Mol Life Sci. 2013 Feb;70(3):459-73. doi: 10.1007/s00018-012-1062-x. Epub 2012 Jul 6. Cell Mol Life Sci. 2013. PMID: 22766974 Free PMC article. Review.
Cited by
- Structural Plasticity of the Hippocampus in Neurodegenerative Diseases.
Weerasinghe-Mudiyanselage PDE, Ang MJ, Kang S, Kim JS, Moon C. Weerasinghe-Mudiyanselage PDE, et al. Int J Mol Sci. 2022 Mar 20;23(6):3349. doi: 10.3390/ijms23063349. Int J Mol Sci. 2022. PMID: 35328770 Free PMC article. Review. - Modeling of Brain-Like Concept Coding with Adulthood Neurogenesis in the Dentate Gyrus.
Wang Y, Gao Y, Deng Y, Yang L. Wang Y, et al. Comput Intell Neurosci. 2019 Nov 3;2019:2367075. doi: 10.1155/2019/2367075. eCollection 2019. Comput Intell Neurosci. 2019. PMID: 31814816 Free PMC article. - Retracted: Allopregnanolone restores the tyrosine hydroxylase-positive neurons and motor performance in a 6-OHDA-injected mouse model.
Chen ZC, Wang TT, Bian W, Ye X, Li MY, Du JJ, Zhou P, Cui HR, Ding YQ, Ren YH, Qi SS, Yuan YY, Liao M, Sun CY. Chen ZC, et al. CNS Neurosci Ther. 2020 Oct;26(10):1069-1082. doi: 10.1111/cns.13432. Epub 2020 Jun 30. CNS Neurosci Ther. 2020. PMID: 32602622 Free PMC article. Retracted. - Synaptic Regulator α-Synuclein in Dopaminergic Fibers Is Essentially Required for the Maintenance of Subependymal Neural Stem Cells.
Perez-Villalba A, Sirerol-Piquer MS, Belenguer G, Soriano-Cantón R, Muñoz-Manchado AB, Villadiego J, Alarcón-Arís D, Soria FN, Dehay B, Bezard E, Vila M, Bortolozzi A, Toledo-Aral JJ, Pérez-Sánchez F, Fariñas I. Perez-Villalba A, et al. J Neurosci. 2018 Jan 24;38(4):814-825. doi: 10.1523/JNEUROSCI.2276-17.2017. Epub 2017 Dec 7. J Neurosci. 2018. PMID: 29217686 Free PMC article. - Neuronal Circuitry Mechanisms Regulating Adult Mammalian Neurogenesis.
Song J, Olsen RH, Sun J, Ming GL, Song H. Song J, et al. Cold Spring Harb Perspect Biol. 2016 Aug 1;8(8):a018937. doi: 10.1101/cshperspect.a018937. Cold Spring Harb Perspect Biol. 2016. PMID: 27143698 Free PMC article. Review.
References
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. - PubMed
- Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. - PubMed
- Chaudhuri KR, Schapira AHV. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurology, The. 2009;8:464–474. - PubMed
- Curtis MA, Penney EB, Pearson J, Dragunow M, Connor B, Faull RL. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington's disease human brain. Neuroscience. 2005;132:777–788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources