Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism - PubMed (original) (raw)
Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism
Ira J Smith et al. Int J Biochem Cell Biol. 2010 May.
Abstract
Sepsis-induced muscle wasting has severe clinical consequences, including muscle weakness, need for prolonged ventilatory support and stay in the intensive care unit, and delayed ambulation with risk for pulmonary and thromboembolic complications. Understanding molecular mechanisms regulating loss of muscle mass in septic patients therefore has significant clinical implications. Forkhead Box O (FOXO) transcription factors have been implicated in muscle wasting, partly reflecting upregulation of the ubiquitin ligases atrogin-1 and MuRF1. The influence of sepsis on FOXO transcription factors in skeletal muscle is poorly understood. We tested the hypothesis that sepsis upregulates expression and activity of FOXO transcription factors in skeletal muscle by a glucocorticoid-dependent mechanism. Sepsis in rats increased muscle FOXO1 and 3a mRNA and protein levels but did not influence FOXO4 expression. Nuclear FOXO1 levels and DNA binding activity were increased in septic muscle whereas FOXO3a nuclear levels were not increased during sepsis. Sepsis-induced expression of FOXO1 was reduced by the glucocorticoid receptor antagonist RU38486 and treatment of rats with dexamethasone increased FOXO1 mRNA levels suggesting that the expression of FOXO1 is regulated by glucocorticoids. Reducing FOXO1, but not FOXO3a, expression by siRNA in cultured L6 myotubes inhibited dexamethasone-induced atrogin-1 and MuRF1 expression, further supporting a role of FOXO1 in glucocorticoid-regulated muscle wasting. Results suggest that sepsis increases FOXO1 expression and activity in skeletal muscle by a glucocorticoid-dependent mechanism and that glucocorticoid-dependent upregulation of atrogin-1 and MuRF1 in skeletal muscle is regulated by FOXO1. The study is significant because it provides novel information about molecular mechanisms involved in sepsis-induced muscle wasting.
2010 Elsevier Ltd. All rights reserved.
Figures
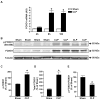
Figure 1. Sepsis increases the expression of FOXO1 in rat skeletal muscle
(A) mRNA levels (arbitrary units, AU) in EDL muscles determined by real-time PCR 4, 8, and 16 h after cecal ligation and puncture (CLP) or sham-operation. (B) Representative Western blots of proteins from EDL muscles for phosphorylated (Ser 256) FOXO1 (p-FOXO1), total FOXO1 and α-tubulin in EDL muscles 16 h after CLP or sham-operation. (C) Densitometric quantifications of p-FOXO1 and (D) total FOXO1 protein levels normalized to α-tubulin levels. (E) Calculated p-FOXO1/total-FOXO1 ratios. Results are means ± SEM with n = 6–8 in each group. *p< 0.05 vs corresponding Sham group by ANOVA (panel A) or Student’s t-test (panels C–E).
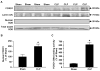
Figure 2. Sepsis increases nuclear FOXO1 protein levels and DNA binding activity in rat skeletal muscle
(A) Representative Western blots of nuclear proteins for FOXO1 and lamin A/C in EDL muscles 16 h after CLP or sham-operation. Nuclear and total tissue extracts were immunoblotted for the cytosolic protein superoxide dismutase (SOD) to validate the nuclear extractions. (B) Densitometric quantification of FOXO1 protein levels normalized to lamin A/C levels. (C) FOXO1 DNA binding activity in nuclear extracts from EDL muscles 16 h after CLP or sham-operation. Results are means ± SEM, with n = 8 in each group. *p<0.05 vs Sham by Student’s t-test.
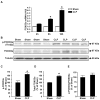
Figure 3. Sepsis increases the expression of FOXO3a in rat skeletal muscle
(A) FOXO3a mRNA levels determined by real-time PCR in EDL muscles 4, 8, and 16 h after CLP or sham-operation. (B) Representative Western blots of proteins from EDL muscles for p(Thr 32)-FOXO3a, total FOXO3a, and α-tubulin 16 h after CLP or sham-operation. (C) Densitometric quantifications of p-FOXO3a and (D) total FOXO3a protein levels normalized to α-tubulin levels. (E) Calculated p-FOXO3a/total-FOXO3 ratios. Results are means ± SEM, with n = 6–8 in each group. *p<0.05 vs corresponding Sham group by ANOVA (panel A) or Student’s t-test (panels C–E).
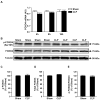
Figure 4. FOXO4 expression is not influenced by sepsis in rat skeletal muscle
(A) FOXO4 mRNA levels determined by real-time PCR in EDL muscles 4, 8, and 16 h after CLP or sham-operation. (B) Representative Western blots of proteins from EDL muscles for p(Ser197)-FOXO4, total FOXO4, and α-tubulin 16 h after CLP or sham-operation. (C) Densitometric quantifications of p(Ser 197)-FOXO4 and (D) total FOXO4 protein levels normalized to α-tubulin levels. (E) Calculated p-FOXO4/total-FOXO4 ratios. Results are means ± SEM, with n = 6–8 in each group.
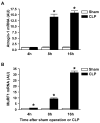
Figure 5. Sepsis increases the expression of atrogin-1 and MuRF1 in rat skeletal muscle
(A) Atrogin-1 and (B) MuRF1 mRNA levels determined by real-time PCR in EDL muscles 4, 8, and 16 h after CLP or sham-operation. Results are means ± SEM with n = 6 in each group. *p<0.05 vs corresponding Sham group by ANOVA.
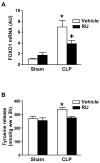
Figure 6. Sepsis-induced changes in FOXO1 expression and protein degradation in skeletal muscle are glucocorticoid-dependent
Sham-operated and septic (CLP) rats were treated with RU-38486 (RU, 10 mg/kg) or corresponding volume (0.3 ml) of vehicle administered intraperitoneally 2 h before sham-operation or CLP. (A) EDL muscles were harvested 16 h after CLP or sham-operation and FOXO1 mRNA levels were determined by real-time PCR. Results are means ± SEM with n = 8 in each group. (B) In a second set of experiments, protein degradation was determined in incubated EDL muscles 16 h after CLP or sham-operation. Results are means ± SEM with n = 6–8 in each group. *p<0.05 vs all other groups by ANOVA. +p<0.05 vs corresponding sham group by ANOVA.
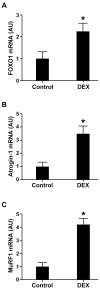
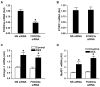
Figure 7. The expression of FOXO1, atrogin-1, and MuRF1 is regulated by glucocorticoids in rat skeletal muscle
Rats were injected with 10 mg/kg of dexamethasone (DEX) intraperitoneally or a corresponding volume (1 ml) of solvent (control). Sixteen hours after injection, EDL muscles were excised and (A) FOXO1, (B) atrogin-1, and (C) MuRF1 mRNA levels were determined by real-time PCR. Results are means ± SEM with n = 7 in each group. *p<0.05 vs corresponding control group by Student’s t-test.
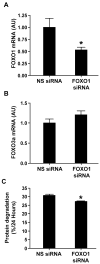
Figure 8. FOXO1 siRNA reduces FOXO1 mRNA levels and protein degradation in cultured L6 myotubes
Myotubes were transfected with non-targeting (non-specific, NS) or FOXO1 siRNA followed by determination of (A) FOXO1 mRNA, (B) FOXO3a mRNA, and (C) protein degradation. Results are means ± SEM with n = 12 in each group. *p<0.05 vs NS siRNA by Student’s t-test.
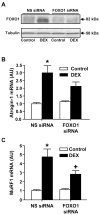
Figure 9. Silencing of FOXO1 in myotubes attenuates dexamethasone-induced increase in FOXO1 protein levels and expression of atrogin-1 and MuRF1
Myotubes were transfected with non-targeting (non-specific, NS) or FOXO1 siRNA, then treated with 1μM dexamethasone or a corresponding concentration of vehicle (control) for 24 h whereafter (A) FOXO1 protein levels were determined by Western blotting and mRNA levels for (B) atrogin-1 and (C) MuRF1 were determined by real-time PCR. In panel (A), each lane was generated by combining tissue from three 10-cm culture dishes. Almost identical results as observed here were seen in a duplicate experiments in which three 10- cm culture dishes were also used for each lane. In panel (B) and (C), results are means ± SEM with n = 12 in each group. *p<0.05 vs corresponding control group by ANOVA. +p<0.05 vs corresponding NS siRNA group by ANOVA.
Figure 10. Silencing of FOXO3a in myotubes does not influence dexamethasone-induced expression of atrogin-1 and MuRF1
Myotubes were transfected with non-targeting (non-specific, NS) or FOXO3a siRNA followed by determination of (A) FOXO3a mRNA and (B) FOXO1 mRNA. Myotubes were then treated with dexamethasone as described in Fig 9, where after mRNA levels for (C) atrogin-1, and (D) MuRF-1 were determined by real-time PCR. Results are means ± SEM, with n = 8–10 in each group. *p<0.05 vs NS siRNA (panel A) by Student’s t-test and vs corresponding control group (panels C and D) by ANOVA.
Similar articles
- PPARβ/δ regulates glucocorticoid- and sepsis-induced FOXO1 activation and muscle wasting.
Castillero E, Alamdari N, Aversa Z, Gurav A, Hasselgren PO. Castillero E, et al. PLoS One. 2013;8(3):e59726. doi: 10.1371/journal.pone.0059726. Epub 2013 Mar 21. PLoS One. 2013. PMID: 23555761 Free PMC article. - Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushing's syndrome.
Kang SH, Lee HA, Kim M, Lee E, Sohn UD, Kim I. Kang SH, et al. Am J Physiol Endocrinol Metab. 2017 Jun 1;312(6):E495-E507. doi: 10.1152/ajpendo.00389.2016. Epub 2017 Feb 28. Am J Physiol Endocrinol Metab. 2017. PMID: 28246104 - Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes.
Castillero E, Alamdari N, Lecker SH, Hasselgren PO. Castillero E, et al. Metabolism. 2013 Oct;62(10):1495-502. doi: 10.1016/j.metabol.2013.05.018. Epub 2013 Jul 15. Metabolism. 2013. PMID: 23866982 - Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy.
Chen K, Gao P, Li Z, Dai A, Yang M, Chen S, Su J, Deng Z, Li L. Chen K, et al. Am J Pathol. 2022 Dec;192(12):1648-1657. doi: 10.1016/j.ajpath.2022.09.003. Epub 2022 Sep 27. Am J Pathol. 2022. PMID: 36174679 Review. - Targeting MuRF1 to Combat Skeletal Muscle Wasting in Cardiac Cachexia: Mechanisms and Therapeutic Prospects.
Liu X, Wen Y, Lu Y. Liu X, et al. Med Sci Monit. 2024 Oct 22;30:e945211. doi: 10.12659/MSM.945211. Med Sci Monit. 2024. PMID: 39434377 Free PMC article. Review.
Cited by
- Hispaglabridin B, a constituent of liquorice identified by a bioinformatics and machine learning approach, relieves protein-energy wasting by inhibiting forkhead box O1.
Huang ZY, Wang LJ, Wang JJ, Feng WJ, Yang ZQ, Ni SH, Huang YS, Li H, Yang Y, Wang MQ, Hu R, Wan H, Wen CJ, Xian SX, Lu L. Huang ZY, et al. Br J Pharmacol. 2019 Jan;176(2):267-281. doi: 10.1111/bph.14508. Epub 2018 Dec 4. Br J Pharmacol. 2019. PMID: 30270561 Free PMC article. - Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients.
Jespersen JG, Nedergaard A, Reitelseder S, Mikkelsen UR, Dideriksen KJ, Agergaard J, Kreiner F, Pott FC, Schjerling P, Kjaer M. Jespersen JG, et al. PLoS One. 2011 Mar 31;6(3):e18090. doi: 10.1371/journal.pone.0018090. PLoS One. 2011. PMID: 21483870 Free PMC article. - Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass.
Goodman CA, Mayhew DL, Hornberger TA. Goodman CA, et al. Cell Signal. 2011 Dec;23(12):1896-906. doi: 10.1016/j.cellsig.2011.07.013. Epub 2011 Jul 23. Cell Signal. 2011. PMID: 21821120 Free PMC article. Review. - Cerium oxide nanoparticle treatment ameliorates peritonitis-induced diaphragm dysfunction.
Asano S, Arvapalli R, Manne ND, Maheshwari M, Ma B, Rice KM, Selvaraj V, Blough ER. Asano S, et al. Int J Nanomedicine. 2015 Oct 5;10:6215-25. doi: 10.2147/IJN.S89783. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26491293 Free PMC article. - The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill.
Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L. Friedrich O, et al. Physiol Rev. 2015 Jul;95(3):1025-109. doi: 10.1152/physrev.00028.2014. Physiol Rev. 2015. PMID: 26133937 Free PMC article. Review.
References
- Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol. 2007;292:C188–99. - PubMed
- Bodine SC, Latres E, Baumheuter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. - PubMed
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FoxO transcription factors by the SirtI deacetylase. Science. 2004;303:2011–5. - PubMed
- Cai D, Frantz JD, Tawa NE, Melendez PA, Oh BC, Lidov HGW, et al. IKKβ/NF-kB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 NR008545-06/NR/NINR NIH HHS/United States
- R01 DK37908/DK/NIDDK NIH HHS/United States
- R56 NR008545/NR/NINR NIH HHS/United States
- R01 NR08545/NR/NINR NIH HHS/United States
- R01 DK037908/DK/NIDDK NIH HHS/United States
- R01 NR008545/NR/NINR NIH HHS/United States
- R01 DK037908-20/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous