Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis - PubMed (original) (raw)
. 2010 Feb 9;107(6):2485-90.
doi: 10.1073/pnas.0908133107. Epub 2010 Jan 13.
Crystal Gore, Jun Liu, Rey-Chen Pong, Ralph Mason, Guiyang Hao, Michael Long, Wareef Kabbani, Luyang Yu, Haifeng Zhang, Hong Chen, Xiankai Sun, David A Boothman, Wang Min, Jer-Tsong Hsieh
Affiliations
- PMID: 20080667
- PMCID: PMC2823864
- DOI: 10.1073/pnas.0908133107
Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis
Daxing Xie et al. Proc Natl Acad Sci U S A. 2010.
Abstract
A single nucleotide polymorphism in the DAB2IP gene is associated with risk of aggressive prostate cancer (PCa), and loss of DAB2IP expression is frequently detected in metastatic PCa. However, the functional role of DAB2IP in PCa remains unknown. Here, we show that the loss of DAB2IP expression initiates epithelial-to-mesenchymal transition (EMT), which is visualized by repression of E-cadherin and up-regulation of vimentin in both human normal prostate epithelial and prostate carcinoma cells as well as in clinical prostate-cancer specimens. Conversely, restoring DAB2IP in metastatic PCa cells reversed EMT. In DAB2IP knockout mice, prostate epithelial cells exhibited elevated mesenchymal markers, which is characteristic of EMT. Using a human prostate xenograft-mouse model, we observed that knocking down endogenous DAB2IP in human carcinoma cells led to the development of multiple lymph node and distant organ metastases. Moreover, we showed that DAB2IP functions as a scaffold protein in regulating EMT by modulating nuclear beta-catenin/T-cell factor activity. These results show the mechanism of DAB2IP in EMT and suggest that assessment of DAB2IP may provide a prognostic biomarker and potential therapeutic target for PCa metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
DAB2IP regulates EMT in various cell lines. (A) Elevated DAB2IP reverses EMT in vitro. The morphology of C4-2 cells transfected with either control vector pCI-neo (Neo) or pCI-DAB2IP (D1, D2) was revealed by phase-contrast microscopy (magnification: 100×). (B) Expression of epithelial or mesenchymal markers in C4-2 (Neo, D1, and D2) sublines was analyzed by Western blotting. β-Actin was used as a loading control. (C) Effect of DAB2IP on cell migration in vitro. Neo, D1, and D2 cells were plated in Transwell chambers for 48 h, and quantitative measurements of migratory cells were determined. Data were presented as mean ± SEM of each sample measured in triplicate. (D) Knockdown of DAB2IP initiates EMT in vitro. PZ-HPV-7 and RWPE-1 cells were infected with control lentivirus or lentivirus-expressing shRNA specific to DAB2IP and then selected with puromycin. Morphology was revealed by phase-contrast microscopy (magnification: 100×). (E) Increased mesenchymal and reduced epithelial markers in DAB2IP-knockdown cells were analyzed by Western blotting.
Fig. 2.
DAB2IP activates GSK-3β and antagonizes Wnt-mediated EMT. (A) DAB2IP prevents β-catenin nuclear translocation. B16 mouse melanoma cells were transfected with control or DAB2IP-siRNA. Subcellular localization of β-catenin was visualized by confocal microscopy (magnification: 500×). (B) DAB2IP inhibits GSK-3β phosphorylation at S9. C4-2 sublines were treated with LiCl (20 mM) for 6 h, and cell lysates were blotted with p-GSK-3β (S9) and GSK-3β antibodies. β-Actin was used as a loading control. (C) DAB2IP inhibits β-catenin/TCF transcriptional activity. C4-2 sublines were transfected with TCF-responsive promoter reporter (TOP-flash) or nonresponsive control reporter (FOP-flash) followed by LiCl treatment (20 mM; 6 h); then, luciferase activity was measured by the ratio of TOP and FOP. Relative luciferase activity is represented as mean ± SEM from each sample after normalizing with control (=1). Asterisk indicates statistical significance in Neo versus D2 cells (P < 0.01), as well as in D2 with versus without LiCl treatment (P < 0.01). (D) DAB2IP activates GSK-3β kinase activity. After treating C4-2 sublines with L- or Wnt-CM, GSK-3β was immunoprecipitated and kinase activities were determined as described in Materials and Methods. Relative GSK-3β kinase activities was represented as mean ± SEM from each sample after normalizing with untreated C4-2 (Neo; =1). Asterisks indicated statistical significance in Neo versus D2 cells (P < 0.01). (E) Knockdown of DAB2IP inactivates GSK-3β, promotes β-catenin activity, and enhances Wnt-induced EMT in PZ-HPV-7 cells. Cells were cotransfected with either control or DAB2IP-siRNA and TOP or FOP and then treated with L- or Wnt-CM. Cell lysates were subjected to Western blot. Relative luciferase analysis was performed as described above. Asterisk
s
indicate statistical significance in cells transfected with control-siRNA cell versus DAB2IP-siRNA (P < 0.05).
Fig. 3.
PP2A is critical for DAB2IP-modulated GSK-3β-β-catenin signaling and EMT. (A) DAB2IP complexes are associated with both GSK3β and PP2A. Two hundred ninety-three cells were transfected with flag control vector (VC) or DAB2IP-F. Cell lysates were immunoprecipitated with GSK-3β, PP2A, and Flag antibodies and then probed with Flag and PP2A or GSK3β antibodies, respectively. (B) Schematic depiction of DAB2IP constructs containing different functional domains and mutants. (C) The effect of C2 domain of DAB2IP on β-catenin/TCF transcription activity. Two hundred ninety-three cells were cotransfected with various DAB2IP transfectants and TOP followed by L-CM or Wnt-CM treatment, and then, luciferase activity was determined. Asterisk indicates statistical significance in 293 cells transfected with VC versus F, N, and PHC2 domain (P < 0.01). (D) Endogenous PP2AC is critical for DAB2IP-mediated S9 phosphorylation of GSK-3β. Two hundred ninety-three cells transfected with either VC or DAB2IP were treated with OA (25 nM, 24 h) or LiCl (20 mM, 6 h) or cotransfected with PP2A-specific siRNA (100 pmol; 24 h). Cell lysates were subjected to Western blotting. (E) The role of PP2A in DAB2IP-modulated EMT. Two hundred ninety-three cells transfected with either VC or DAB2IP were cotransfected with PP2A-specific siRNA. Cell lysates were subjected to Western blotting.
Fig. 4.
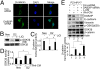
DAB2IP down-regulation promotes tumor growth and metastasis. (A) Representative BLI imaging of mice bearing PC-3-KD1 tumors with multiple metastatic lesions. Mice (n = 9) were imaged 10 days later to determine local tumor growth and metastasis. (B) Numbers of metastatic nodules in individual mouse bearing Con or KD1 tumors at the time of sacrifice. (C) Representative H&E and IHC staining patterns. H&E staining showed primary tumor without detectable metastases in control mice and the lymph node metastases in mice bearing KD1 tumors 2 weeks postinjection (magnification: 100×). IHC showed the majority of KD1 tumors with a strong positive vimentin staining but weak E-cadherin and Cytokeratin staining (magnification: 200×). The dashed line separates between the area of mouse prostate (P) and PC-3 tumor (T).
Fig. 5.
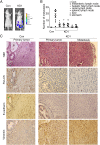
DAB2IP KO mice exhibit mesenchymal characteristics in the prostate gland. (A) DAB2IP−/− mice express elevated mesenchymal markers in the prostate gland. Expression of DAB2IP, E-cadherin, Vimentin, and β-catenin in paraffin sections of prostate were determined by IHC (magnification: 400×). (B) Reduced GSK-3β activity in DAB2IP−/− mice. Prostate homogenate from WT and KO mice were subjected to Western blotting. (C) Loss of DAB2IP expression correlates with EMT marker changes in clinical specimens of prostate-cancer patients. Expression levels of DAB2IP, E-cadherin, vimentin, and β-catenin protein as well as p-GSK-3β(S9) levels in normal (n = 6), BPH (n = 6), and PCa (n = 10) tissues were determined by Western blotting. Densitometry was normalized with β-Actin level.
Comment in
- ASK-ing EMT not to spread cancer.
Kyprianou N. Kyprianou N. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):2731-2. doi: 10.1073/pnas.0914721107. Epub 2010 Feb 9. Proc Natl Acad Sci U S A. 2010. PMID: 20145111 Free PMC article. No abstract available.
Similar articles
- The involvement of FBP1 in prostate cancer cell epithelial mesenchymal transition, invasion and metastasis by regulating the MAPK signaling pathway.
Zhang YP, Liu KL, Yang Z, Lu BS, Qi JC, Han ZW, Yin YW, Zhang M, Chen DM, Wang XW, Li W, Xin H. Zhang YP, et al. Cell Cycle. 2019 Oct;18(19):2432-2446. doi: 10.1080/15384101.2019.1648956. Epub 2019 Aug 25. Cell Cycle. 2019. PMID: 31448674 Free PMC article. Retracted. - DAB2IP regulates EMT and metastasis of prostate cancer through targeting PROX1 transcription and destabilizing HIF1α protein.
Wang B, Huang J, Zhou J, Hui K, Xu S, Fan J, Li L, Wang X, Hsieh JT, He D, Wu K. Wang B, et al. Cell Signal. 2016 Nov;28(11):1623-30. doi: 10.1016/j.cellsig.2016.07.011. Epub 2016 Jul 27. Cell Signal. 2016. PMID: 27476001 - The function of SARI in modulating epithelial-mesenchymal transition and lung adenocarcinoma metastasis.
Wang C, Su Y, Zhang L, Wang M, You J, Zhao X, Zhang Z, Liu J, Hao X. Wang C, et al. PLoS One. 2012;7(9):e38046. doi: 10.1371/journal.pone.0038046. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23049725 Free PMC article. - An update on the tumor-suppressive functions of the RasGAP protein DAB2IP with focus on therapeutic implications.
De Florian Fania R, Bellazzo A, Collavin L. De Florian Fania R, et al. Cell Death Differ. 2024 Jul;31(7):844-854. doi: 10.1038/s41418-024-01332-3. Epub 2024 Jun 20. Cell Death Differ. 2024. PMID: 38902547 Free PMC article. Review. - E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas.
Wells A, Yates C, Shepard CR. Wells A, et al. Clin Exp Metastasis. 2008;25(6):621-8. doi: 10.1007/s10585-008-9167-1. Epub 2008 Jul 4. Clin Exp Metastasis. 2008. PMID: 18600305 Free PMC article. Review.
Cited by
- DAB2IP Expression in Abdominal Aortic Aneurysm: EZH2 and mir-363-3p as Potential Mediators.
Legaki E, Klonaris C, Athanasiadis D, Patelis N, Sioziou A, Liakakos T, Gazouli M. Legaki E, et al. In Vivo. 2019 May-Jun;33(3):737-742. doi: 10.21873/invivo.11533. In Vivo. 2019. PMID: 31028191 Free PMC article. - DAB2IP in cancer.
Liu L, Xu C, Hsieh JT, Gong J, Xie D. Liu L, et al. Oncotarget. 2016 Jan 26;7(4):3766-76. doi: 10.18632/oncotarget.6501. Oncotarget. 2016. PMID: 26658103 Free PMC article. Review. - The positive feedback between Snail and DAB2IP regulates EMT, invasion and metastasis in colorectal cancer.
Wang J, Zhu X, Hu J, He G, Li X, Wu P, Ren X, Wang F, Liao W, Liang L, Ding Y. Wang J, et al. Oncotarget. 2015 Sep 29;6(29):27427-39. doi: 10.18632/oncotarget.4861. Oncotarget. 2015. PMID: 26336990 Free PMC article. - Cell-autonomous and cell non-autonomous downregulation of tumor suppressor DAB2IP by microRNA-149-3p promotes aggressiveness of cancer cells.
Bellazzo A, Di Minin G, Valentino E, Sicari D, Torre D, Marchionni L, Serpi F, Stadler MB, Taverna D, Zuccolotto G, Montagner IM, Rosato A, Tonon F, Zennaro C, Agostinis C, Bulla R, Mano M, Del Sal G, Collavin L. Bellazzo A, et al. Cell Death Differ. 2018 Jul;25(7):1224-1238. doi: 10.1038/s41418-018-0088-5. Epub 2018 Mar 22. Cell Death Differ. 2018. PMID: 29568059 Free PMC article. - Epigenetic Regulation by Lysine Demethylase 5 (KDM5) Enzymes in Cancer.
Blair LP, Cao J, Zou MR, Sayegh J, Yan Q. Blair LP, et al. Cancers (Basel). 2011 Mar 1;3(1):1383-404. doi: 10.3390/cancers3011383. Cancers (Basel). 2011. PMID: 21544224 Free PMC article.
References
- Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. - PubMed
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. - PubMed
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. - PubMed
- Tomita K, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–3654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA102792/CA/NCI NIH HHS/United States
- U24CA126608/CA/NCI NIH HHS/United States
- P30 CA142543/CA/NCI NIH HHS/United States
- 5U19AI067773-04/AI/NIAID NIH HHS/United States
- U19 AI067773/AI/NIAID NIH HHS/United States
- U24 CA126608/CA/NCI NIH HHS/United States
- R01 CA139217/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases