Lipid-like materials for low-dose, in vivo gene silencing - PubMed (original) (raw)
. 2010 Feb 2;107(5):1864-9.
doi: 10.1073/pnas.0910603106. Epub 2010 Jan 11.
Kerry P Mahon, Christopher G Levins, Kathryn A Whitehead, William Querbes, J Robert Dorkin, June Qin, William Cantley, Liu Liang Qin, Timothy Racie, Maria Frank-Kamenetsky, Ka Ning Yip, Rene Alvarez, Dinah W Y Sah, Antonin de Fougerolles, Kevin Fitzgerald, Victor Koteliansky, Akin Akinc, Robert Langer, Daniel G Anderson
Affiliations
- PMID: 20080679
- PMCID: PMC2804742
- DOI: 10.1073/pnas.0910603106
Lipid-like materials for low-dose, in vivo gene silencing
Kevin T Love et al. Proc Natl Acad Sci U S A. 2010.
Erratum in
- Proc Natl Acad Sci U S A. 2010 May 25;107(21):9915
Abstract
Significant effort has been applied to discover and develop vehicles which can guide small interfering RNAs (siRNA) through the many barriers guarding the interior of target cells. While studies have demonstrated the potential of gene silencing in vivo, improvements in delivery efficacy are required to fulfill the broadest potential of RNA interference therapeutics. Through the combinatorial synthesis and screening of a different class of materials, a formulation has been identified that enables siRNA-directed liver gene silencing in mice at doses below 0.01 mg/kg. This formulation was also shown to specifically inhibit expression of five hepatic genes simultaneously, after a single injection. The potential of this formulation was further validated in nonhuman primates, where high levels of knockdown of the clinically relevant gene transthyretin was observed at doses as low as 0.03 mg/kg. To our knowledge, this formulation facilitates gene silencing at orders-of-magnitude lower doses than required by any previously described siRNA liver delivery system.
Conflict of interest statement
Confllict of interest statement: The Sponsor declares a conflict of interest. R.L. is a shareholder and member of the Scientific Advisory Board of Alnylam. D.G.A. is a consultant with Alnylam Pharmaceuticals. R.L and D.G.A have sponsored research grants from Alnylam. Alnylam also has a license to certain intellectual property invented at Massachusetts Institute of Technology by Drs. Anderson, Langer, and colleagues. W.Q., J.R.D., J.Q., W.C., L.L.Q., T.R., M.F.-K., K.F., V.K., A.F., R.A., D.W.Y.S., and A.A. are employed by Alnylam. The authors declare a conflict of interest. R.L. is a shareholder and member of the Scientific Advisory Board of Alnylam. D.G.A. is a consultant with Alnylam Pharmaceuticals. R.L and D.G.A have sponsored research grants from Alnylam. Alnylam also has a license to certain intellectual property invented at Massachusetts Institute of Technology by Drs. Anderson, Langer, and colleagues. W.Q., J.R.D., J.Q., W.C., L.L.Q., T.R., M.F.-K., K.F., V.K., and A.A. are employed by Alnylam.
Figures
Fig. 1.
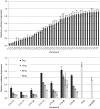
Synthesis of Epoxide-Derived Lipidoid Library (A) Epoxide terminated alkyl chains and amine-containing monomers were used in synthesis of combinatorial library, (B) addition of epoxides to amines by efficient ring-opening enables parallel synthesis of library members
Fig. 2.
In vitro screening of lipidoid library. Lipidoids were screened in luciferase-expressing Hela-derived cell line. (A) Antifirefly luciferase siRNA was complexed with lipidoids and incubated with cells in presence of growth media. Relative firefly luciferase expression determined by comparison of detected protein levels in treated groups vs. untreated control. (B) Luciferase silencing at low doses of siRNA (s.d., n = 4)
Fig. 3.
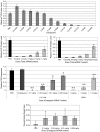
In vivo silencing of Factor VII in mice. A) Top-performing lipidoids from in vitro screen were purified, formulated for serum stability and delivered intravenously to C57BL/6 mice. Mice received a single bolus administration of 3 mg/kg total siRNA via tail-vein injection and Factor VII levels were quantified 72 h postinjection. B_–_D) Dose response experiments with top three performing lipidoids from in vivo screen; C16-96 (B), C14-110 (C), and C12-200 (D). No lipidoid-related toxicity is observed as measured by body-weight loss (E). (s.d.,n = 3 or 4, * P < 0.005, **P < 0.001; t-test, single tailed)
Fig. 4.
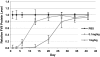
In vivo persistence of C12-200-mediated silencing was investigated by monitoring Factor VII protein levels for a period of over 40 days. Mice we administered a single dose of either 1 or 0.1 mg/kg siRNA and blood samples were drawn at varying timepoints for quantitation of serum protein levels. (s.d.,n = 3)
Fig. 5.
Five hepatocellular gene targets were simultaneously silenced by a single injection of pooled siRNAs formulated with C12-200. Mice we administered a single dose and dosage was titrated from 0.2 and .005 mg/kg per siRNA. 72 h postinjection, liver tissue was harvested for analysis of gene transcript levels. (s.d.,n = 5).
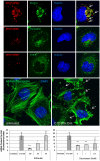
Fig. 6.
C12-200-siRNA particles elicit cellular uptake by macropinocytosis. (A) Fluorescently labeled siRNA formulated with C12-200 was incubated with HeLa cells in the presence of labeled markers of various endocytic pathways. siRNA-containing particles colocalize with labeled dextran, a fluid phase marker known to enter cells via macropinocytosis (White arrows). (B) Fluorescence labeling of actin fibers reveals membrane ruffling (White arrows) and actin rearrangement, hallmark indicators of uptake by macropinocytosis, within 15 min of exposure of HeLa cells to C12-200-siRNA particles. (C,D) Prior exposure of cells to EIPA and Cytochalasin D, inhibitors of macropinocytosis and actin polymerization, respectively, reduce uptake of C12-200-siRNA particles in dose-dependent fashion. (s.d., n = 3, *** P < 0.005; ** P < 0.01; * P < 0.05
Fig. 7.
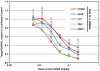
Efficacy of C12-200 in nonhuman primates. Cynomolgus monkeys (n = 3 per group) received either PBS or 0.03, 0.1, or 0.3 mg/kg siTTR formulated in C12-200 as 15 min intravenous infusions (5 mL/kg) via the cephalic vein. Liver biopsies were collected from animals at 48 h postadministration. TTR mRNA levels relative to GAPDH mRNA levels were determined in liver samples. Data points represent group mean ± s.d
Similar articles
- Development of lipidoid-siRNA formulations for systemic delivery to the liver.
Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, Jayaprakash KN, Jayaraman M, Rajeev KG, Manoharan M, Koteliansky V, Röhl I, Leshchiner ES, Langer R, Anderson DG. Akinc A, et al. Mol Ther. 2009 May;17(5):872-9. doi: 10.1038/mt.2009.36. Epub 2009 Mar 3. Mol Ther. 2009. PMID: 19259063 Free PMC article. - Lipid Nanoparticle Formulations for Enhanced Co-delivery of siRNA and mRNA.
Ball RL, Hajj KA, Vizelman J, Bajaj P, Whitehead KA. Ball RL, et al. Nano Lett. 2018 Jun 13;18(6):3814-3822. doi: 10.1021/acs.nanolett.8b01101. Epub 2018 May 8. Nano Lett. 2018. PMID: 29694050 - Effect of PEGylation on biodistribution and gene silencing of siRNA/lipid nanoparticle complexes.
Bao Y, Jin Y, Chivukula P, Zhang J, Liu Y, Liu J, Clamme JP, Mahato RI, Ng D, Ying W, Wang Y, Yu L. Bao Y, et al. Pharm Res. 2013 Feb;30(2):342-51. doi: 10.1007/s11095-012-0874-6. Epub 2012 Sep 15. Pharm Res. 2013. PMID: 22983644 - Lipid nanoparticles for short interfering RNA delivery.
Leung AK, Tam YY, Cullis PR. Leung AK, et al. Adv Genet. 2014;88:71-110. doi: 10.1016/B978-0-12-800148-6.00004-3. Adv Genet. 2014. PMID: 25409604 Free PMC article. Review. - Lipid Nanoparticle Systems for Enabling Gene Therapies.
Cullis PR, Hope MJ. Cullis PR, et al. Mol Ther. 2017 Jul 5;25(7):1467-1475. doi: 10.1016/j.ymthe.2017.03.013. Epub 2017 Apr 13. Mol Ther. 2017. PMID: 28412170 Free PMC article. Review.
Cited by
- Rationale and Application of PEGylated Lipid-Based System for Advanced Target Delivery of siRNA.
Ge X, Chen L, Zhao B, Yuan W. Ge X, et al. Front Pharmacol. 2021 Jan 20;11:598175. doi: 10.3389/fphar.2020.598175. eCollection 2020. Front Pharmacol. 2021. PMID: 33716725 Free PMC article. Review. - Fast and facile synthesis of amidine-incorporated degradable lipids for versatile mRNA delivery in vivo.
Han X, Alameh MG, Gong N, Xue L, Ghattas M, Bojja G, Xu J, Zhao G, Warzecha CC, Padilla MS, El-Mayta R, Dwivedi G, Xu Y, Vaughan AE, Wilson JM, Weissman D, Mitchell MJ. Han X, et al. Nat Chem. 2024 Oct;16(10):1687-1697. doi: 10.1038/s41557-024-01557-2. Epub 2024 Jul 9. Nat Chem. 2024. PMID: 38982196 Free PMC article. - Lipid nanovehicles overcome barriers to systemic RNA delivery: Lipid components, fabrication methods, and rational design.
Yan J, Zhang H, Li G, Su J, Wei Y, Xu C. Yan J, et al. Acta Pharm Sin B. 2024 Feb;14(2):579-601. doi: 10.1016/j.apsb.2023.10.012. Epub 2023 Oct 20. Acta Pharm Sin B. 2024. PMID: 38322344 Free PMC article. Review. - Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system.
DeRosa F, Guild B, Karve S, Smith L, Love K, Dorkin JR, Kauffman KJ, Zhang J, Yahalom B, Anderson DG, Heartlein MW. DeRosa F, et al. Gene Ther. 2016 Oct;23(10):699-707. doi: 10.1038/gt.2016.46. Epub 2016 Jun 30. Gene Ther. 2016. PMID: 27356951 Free PMC article.
References
- Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
- Vargason JM, Szittya G, Burgyan J, Hall TM. Size selective recognition of siRNA by an RNA silencing suppressor. Cell. 2003;115:799–811. - PubMed
- Judge AD, et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials