Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production - PubMed (original) (raw)
. 2010 Jan 26;107(4):1576-81.
doi: 10.1073/pnas.0912344107. Epub 2010 Jan 4.
XueQing Lun, Yvan Martineau, Polen Sean, Bali Pulendran, Emmanuel Petroulakis, Franz J Zemp, Chantal G Lemay, Dominic Roy, John C Bell, George Thomas, Sara C Kozma, Peter A Forsyth, Mauro Costa-Mattioli, Nahum Sonenberg
Affiliations
- PMID: 20080710
- PMCID: PMC2824402
- DOI: 10.1073/pnas.0912344107
Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production
Tommy Alain et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Oncolytic viruses constitute a promising therapy against malignant gliomas (MGs). However, virus-induced type I IFN greatly limits its clinical application. The kinase mammalian target of rapamycin (mTOR) stimulates type I IFN production via phosphorylation of its effector proteins, 4E-BPs and S6Ks. Here we show that mouse embryonic fibroblasts and mice lacking S6K1 and S6K2 are more susceptible to vesicular stomatitis virus (VSV) infection than their WT counterparts as a result of an impaired type I IFN response. We used this knowledge to employ a pharmacoviral approach to treat MGs. The highly specific inhibitor of mTOR rapamycin, in combination with an IFN-sensitive VSV-mutant strain (VSV(DeltaM51)), dramatically increased the survival of immunocompetent rats bearing MGs. More importantly, VSV(DeltaM51) selectively killed tumor, but not normal cells, in MG-bearing rats treated with rapamycin. These results demonstrate that reducing type I IFNs through inhibition of mTORC1 is an effective strategy to augment the therapeutic activity of VSV(DeltaM51).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
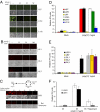
Glioma cell lines are responsive to and produce type I IFN. (A) Addition of IFN-β protects glioma cells against VSV infection. The human glioma cell lines U87, U251, and U118 were pretreated with increasing amounts of human IFN-β for 6 h before infection with VSVΔM51-GFP (MOI of 1). At 24 h after infection, GFP fluorescence and CPE were analyzed by phase-contrast and fluorescent microscopy. (B) Freshly excised glioma cells were treated as described and infected with VSVΔM51-RFP (MOI of 10). (C) Poly(I:C) stimulates type I IFN in RG2 cells and conditioned media protect cells against VSV infection. Scheme of the assay: RG2 cells were transfected with poly(I:C) dsRNA (1 μg/mL) for 6 h. Cultured medium was used to treat either RG2 or Rat astrocytes for 12 h before infection with VSVΔM51-RFP (MOI of 1). RFP fluorescence and CPE were analyzed as described. (D) Detection of type I IFN production. Human foreskin fibroblast and the indicated human glioma cell lines were treated with poly(I:C) (1 μg/mL) for 24 h. Supernatant was collected and 30 μL was used to condition HEK-Blue type I IFN cells (InvivoGen). OD was measured by colorimetric assay at 650 nm using the Quanti-Blue reagent (InvivoGen). IFN production was plotted as relative IFN units. (E) Freshly excised glioma cells were treated with poly(I:C) (1 μg/mL) for 24 h and type I IFN production was assessed as in D. (F) Rapamycin reduces the type I IFN response in glioma cells. Human glioma cell lines U251 and U343 were pretreated with DMSO or rapamycin (20 nM) for 1 h before poly(I:C) stimulation at 1 μg/mL, and HEK-Blue type I IFN colorimetric assay was performed 6 h after poly(I:C) stimulation.
Fig 2.
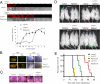
Rapamycin increases VSVΔM51 oncolysis by reducing type I IFN production. Rapamycin reduces the level of antiviral cytokines that is generated upon VSVΔM51 infection. (A) Rats were treated or untreated with rapamycin (5 mg/kg) 1 d before and every day after i.v. infection with VSVΔM51-GFP (MOI of 5 × 108 pfu per rat). At 48 h after infection, sera were collected and different dilutions were added to RG2 cells for 1 h before infection with VSVΔM51-RFP (MOI of 0.1). At 20 h after infection, RFP fluorescence and CPE were assessed as in Fig. 1A. Remaining cell viability was further confirmed by MTT assay. (B) Presence of VSV in glioma tumors. GFP fluorescence and immunohistochemistry of VSV proteins on brain slices from rats infected with VSVΔM51-GFP (72 h after infection) pretreated or untreated with rapamycin. The combination of VSVΔM51 and rapamycin prolongs the survival and reduces tumor growth of a rat model of malignant glioma. (C) H&E staining of RG2 tumors from the different conditions on d 13 post RG2 intracranial injection. (D) On d 0, rats were intracranially implanted with RG2 rat glioma cells expressing luciferase (RG2-Luc; 1 × 104 cells). On d 6, rapamycin (5 mg/kg) was administered by i.p. injection for 10 d. Control rats received vehicle only. On d 7, VSVΔM51-GFP or PBS solution was administered by i.v. injection (MOI of 5 × 108 pfu). Photograph showing the luciferase expression of RG2-Luc tumors at d 13, d 17, and d 21 from representative rats of each group. (E) Kaplan-Meier survival curve. Control median survival, 15.6 d; VSVΔM51-GFP, 16.4 d; rapamycin, 22 d; VSVΔM51-GFP + rapamycin, 39.8 d. Control and VSVΔM51-GFP log-rank test, P = 0.2402; control and rapamycin log-rank test, P = 0.0038; control and VSVΔM51-GFP plus rapamycin log-rank test, P = 0.0007; rapamycin and VSVΔM51-GFP plus rapamycin log-rank test, P = 0.0011.
Fig. 3.
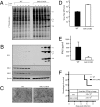
MEFs and mice lacking S6K1/2 are sensitive to VSV infection. WT and S6K1/2 DKO MEFs were infected with VSV (MOI of 10) and viral infection was followed up to 10 h after infection. (A) MEFs were incubated with [35S]methionine for 1 h at the indicated times after infection. Proteins were subjected to SDS-PAGE and transferred to a membrane. An autoradiogram is shown. Viral proteins are indicated on the right. G, glycoprotein; M, matrix protein; N/P, nucleocapsid protein/phosphoprotein. The infection was also confirmed by Western blotting analysis using antibodies against VSV proteins, S6K1, S6K2 and β-actin (B), VSV-induced CPE at 12 h after infection (C), and viral titers determined by plaque assay at 30 h after infection at an MOI of 1 (D) (mean ± SD of three independent experiments). (E) WT and S6K1/2 DKO mice (n = 3 per group) were infected with VSV (1 × 108 pfu) and serum was collected at 48 h after infection. IFN-β levels were measured by ELISA. (F) Survival of WT and S6K1/2 DKO infected with VSV. Mice (n = 10) were intranasally infected with VSV (1 × 108 pfu) and their survival was plotted as a Kaplan-Meier curve. Viral titers found in lung tissues 3 d after infection are shown.
Fig. 4.
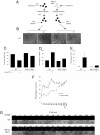
MEFs lacking S6K1/2 have an impaired type I IFN response. (A) Scheme of the protection assay experiment: WT and S6K1/2 DKO MEFs were mock treated or treated with 1 μg/mL of synthetic dsRNA poly(I:C) for a period of 6 h. Supernatants from cells were subsequently collected and put onto WT cells overnight. The next day, WT cells were infected with VSV at an MOI of 1 for 36 h. Viral infections were assessed by cytopathic effects (B), virus titers as determined by plaque assays (C), remaining viability as measured by MTT assay (D), and IFN-α production measured by ELISA (E). (Error bars correspond to the mean ± SD of experiments done in triplicate.) Impaired response to exogenous type I IFN in S6K1/2 DKO MEFs. WT and S6K1/2 DKO MEFs were treated with mouse IFN-α/β for 6 h, followed by infection with VSV-GFP (MOI of 1). Infection was monitored at 36 h after infection by MTT assay (F) and GFP fluorescence and CPE (G).
Similar articles
- Cell Cycle Arrest in G2/M Phase Enhances Replication of Interferon-Sensitive Cytoplasmic RNA Viruses via Inhibition of Antiviral Gene Expression.
Bressy C, Droby GN, Maldonado BD, Steuerwald N, Grdzelishvili VZ. Bressy C, et al. J Virol. 2019 Feb 5;93(4):e01885-18. doi: 10.1128/JVI.01885-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487274 Free PMC article. - Integrin Alpha E (CD103) Limits Virus-Induced IFN-I Production in Conventional Dendritic Cells.
Duhan V, Khairnar V, Kitanovski S, Hamdan TA, Klein AD, Lang J, Ali M, Adomati T, Bhat H, Friedrich SK, Li F, Krebs P, Futerman AH, Addo MM, Hardt C, Hoffmann D, Lang PA, Lang KS. Duhan V, et al. Front Immunol. 2021 Jan 27;11:607889. doi: 10.3389/fimmu.2020.607889. eCollection 2020. Front Immunol. 2021. PMID: 33584680 Free PMC article. - Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas.
Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, Lichty B, Power A, Johnston RN, Hamilton M, Parney I, Bell JC, Forsyth PA. Lun X, et al. J Natl Cancer Inst. 2006 Nov 1;98(21):1546-57. doi: 10.1093/jnci/djj413. J Natl Cancer Inst. 2006. PMID: 17077357 - [Vesicular stomatitis virus in the fight against cancer].
Janelle V, Poliquin L, Lamarre A. Janelle V, et al. Med Sci (Paris). 2013 Feb;29(2):175-82. doi: 10.1051/medsci/2013292015. Epub 2013 Feb 28. Med Sci (Paris). 2013. PMID: 23452604 Review. French. - Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy.
Choo AY, Blenis J. Choo AY, et al. Cell Cycle. 2009 Feb 15;8(4):567-72. doi: 10.4161/cc.8.4.7659. Epub 2009 Feb 18. Cell Cycle. 2009. PMID: 19197153 Review.
Cited by
- Infection by the Protozoan Parasite Toxoplasma gondii Inhibits Host MNK1/2-eIF4E Axis to Promote Its Survival.
Leroux LP, Chaparro V, Jaramillo M. Leroux LP, et al. Front Cell Infect Microbiol. 2020 Sep 9;10:488. doi: 10.3389/fcimb.2020.00488. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33014898 Free PMC article. - Oncolytic virotherapy: the questions and the promise.
Aurelian L. Aurelian L. Oncolytic Virother. 2013 May 31;2:19-29. doi: 10.2147/OV.S39609. eCollection 2013. Oncolytic Virother. 2013. PMID: 27512655 Free PMC article. Review. - HijAkt: The PI3K/Akt pathway in virus replication and pathogenesis.
Dunn EF, Connor JH. Dunn EF, et al. Prog Mol Biol Transl Sci. 2012;106:223-50. doi: 10.1016/B978-0-12-396456-4.00002-X. Prog Mol Biol Transl Sci. 2012. PMID: 22340720 Free PMC article. Review. - Role of MAPK/MNK1 signaling in virus replication.
Kumar R, Khandelwal N, Thachamvally R, Tripathi BN, Barua S, Kashyap SK, Maherchandani S, Kumar N. Kumar R, et al. Virus Res. 2018 Jul 15;253:48-61. doi: 10.1016/j.virusres.2018.05.028. Epub 2018 Jun 1. Virus Res. 2018. PMID: 29864503 Free PMC article. Review. - Pharmacological modulation of anti-tumor immunity induced by oncolytic viruses.
Forbes NE, Krishnan R, Diallo JS. Forbes NE, et al. Front Oncol. 2014 Jul 23;4:191. doi: 10.3389/fonc.2014.00191. eCollection 2014. Front Oncol. 2014. PMID: 25101247 Free PMC article. Review.
References
- Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. - PubMed
- Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–1378. - PubMed
- Shai R, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22:4918–4923. - PubMed
- Stupp R, van den Bent MJ, Hegi ME. Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5:198–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous