Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition - PubMed (original) (raw)
Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition
Andrea Rasola et al. Proc Natl Acad Sci U S A. 2010.
Abstract
We studied human cancer cell models in which we detected constitutive activation of ERK. A fraction of active ERK was found to be located in mitochondria in RWPE-2 cells, obtained by v-Ki-Ras transformation of the epithelial prostate RWPE-1 cell line; in metastatic prostate cancer DU145 cells; and in osteosarcoma SAOS-2 cells. All these tumor cells displayed marked resistance to death caused by apoptotic stimuli like arachidonic acid and the BH3 mimetic EM20-25, which cause cell death through the mitochondrial permeability transition pore (PTP). PTP desensitization and the ensuing resistance to cell death induced by arachidonic acid or EM20-25 could be ablated by inhibiting ERK with the drug PD98059 or with a selective ERK activation inhibitor peptide. ERK inhibition enhanced glycogen synthase kinase-3 (GSK-3)-dependent phosphorylation of the pore regulator cyclophilin D, whereas GSK-3 inhibition protected from PTP opening. Neither active ERK in mitochondria nor pore desensitization was observed in non-transformed RWPE-1 cells. Thus, in tumor cells mitochondrial ERK activation desensitizes the PTP through a signaling axis that involves GSK-3 and cyclophilin D, a finding that provides a mechanistic basis for increased resistance to apoptosis of neoplastic cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
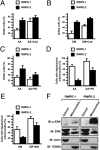
RWPE-2 cells are more resistant than RWPE-1 cells to PTP agonists and are sensitized by ERK inhibition. (A–C) Viable cells are identified as double negative for propidium iodide and annexin V–FITC in FACS analyses. (D and E) Cells with depolarized mitochondria are assessed by FACS analysis after TMRM staining. RWPE-1/2 cells were exposed for 1 h to 50 μM arachidonic acid (AA) (A, C, and D) or to 500 μM EM20-25 (EM; B and E). CsA (4 μM), PD98059 (PD, 40 μM), or ERK inhibitor peptide (EIP) (50 μM) were preincubated for 30 min. In all histograms, bars are mean values of percentages (±SD). (F) Subcellular distribution of phospho- and total ERK was assessed by Western immunoblot (IB) in the mitochondrial and cytosolic fractions of RWPE-1/2 cells. Blots were probed with an anti-TOM20 as a mitochondrial marker and with an anti-actin as a cytosolic marker.
Fig. 2.
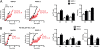
RWPE-2 cells are less sensitive than RWPE-1 cells to pore opening, which is enhanced by ERK inhibition. (A) PTP opening of RWPE-1/2 cells treated with arachidonic acid (AA) (1 μM) or with EM20-25 (EM, 50 μM) is measured with the whole-cell CRC assay. In B, cells were also pretreated with the ERK inhibitor PD98059 (PD, 40 μM). Calcium Green-5N fluorescence is reported as arbitrary units on the y axis. Because the probe does not permeate mitochondria, Ca2+ uptake into the organelles is displayed by a rapid decrease of the fluorescence spike after administration to the cells of subsequent Ca2+ pulses (5 μM each). CsA (red) increases the number of spikes before the permeability transition, recorded as a marked fluorescence increase, occurs. This establishes the PTP dependence of Ca2+ release. In the histograms, both the Ca2+ concentration required to open the pore (Left) and the ratio between the CRC detected in the presence (CRC) and absence (CRC0) of the agonist (Right) are reported.
Fig. 3.
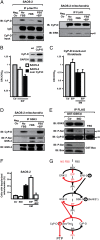
A fraction of ERK and of GSK-3 is located in mitochondria, and ERK inhibition sensitizes SAOS-2 cells to mitochondrial depolarization and PTP opening. (A) Isolated mitochondria (70 μg per point) were treated for 1 h with the reported quantities of trypsin (in micrograms; trypsin concentration: 5, 10, or 20 μg/mL) and analyzed by Western immunoblot (IB). Where indicated, 0.1% SDS was added before trypsin. Blots were probed for ERK and GSK-3; and for a panel of mitochondrial markers of the outer and inner membrane (OMM and IMM, respectively), of the intermembrane space (IMS), and of the matrix. COX-IV is a cytochrome oxidase subunit. Poly(ADP-ribose)polymerase (PARP) was used as an example of a non-mitochondrial soluble protein; and calnexin (CNX) as an endoplasmic reticulum marker. Densitometry analyzes ERK or GSK-3 levels. (B) Ca2+ uptake in trypsin-treated mitochondria was assessed by Calcium Green-5N fluorescence. Because the probe does not permeate mitochondria, Ca2+ entering viable organelles is displayed by a rapid decrease of the fluorescence spike after administration to the cells of subsequent Ca2+ pulses (5 μM each). The final increase in fluorescence indicates pore opening. (C) FACS analysis [forward scatter (FSC) vs. TMRM] showing mitochondria depolarization in cells exposed to EM20-25 (EM) for 1 h with or without a 30-min preincubation with EIP. The percentage of viable cells (V, TMRM positive, in the quadrant) is reported. (D) PTP opening was measured as in B, but on whole cells. Treatment with ERK inhibitor peptide (EIP) (5 μM, in red) decreased the number of spikes before the permeability transition occurred, either in control conditions or when cells were incubated with EM20-25 (EM, 50 μM).
Fig. 4.
ERK activity modulates mitochondrial depolarization and CyP-D phosphorylation through GSK-3. (A) Immunoprecipitations (IP) on lysates of SAOS-2 cells starved overnight (No FBS) and then stimulated for 15 min with serum with or without a 30 min preincubation with EIP (50 μM; lanes FBS and FBS+EIP). Left: P-Ser/Thr were immunoprecipitated from total cell lysates; coimmunoprecipitation of CyP-D is shown, and IgG and input of CyP-D are reported as loading control. Right: the FLAG peptide was immunoprecipitated from mitochondria of SAOS-2 cells expressing FLAG-CyP-D. Immunoprecipitation of CyP-D and coimmunoprecipitation of ERK2 (p42) are shown. The negative control was a SAOS-2 lysate incubated with the nonconjugated resin (Left) or a lysate of SAOS-2 wild-type mitochondria incubated with the FLAG-conjugated resin (Right). (B and C) Ratio between CRC detected in the presence (CRC) and absence (CRC0) of EIP are reported for wild-type or CyP-D-overexpressing SAOS-2 cells (B), or for CyP-D knockout fibroblasts (C), which were also treated with EM20-25 (EM). Numbers are mean values of four experiments (±SD). (D) GSK-3 was immunoprecipitated from SAOS-2 mitochondria. Coimmunoprecipitation of CyP-D and Ser-phosphorylation of GSK-3 are shown. The negative control was as in A (Left). (E) In vitro CyP-D phosphorylation by recombinant GST-GSK-3β, without or with the GSK-3 inhibitor indirubin-3′-oxime (IND, 3 μM). The FLAG peptide was immunoprecipitated from lysates of SAOS-2 cells expressing FLAG-CyP-D. GST-Bax was used as a positive control for enzyme activity. Right: Ser phosphorylation was analyzed on the same quantity of FLAG-CyP-D immunoprecipitated from cells kept with serum. (F) Mitochondria depolarization of SAOS-2 cells exposed to 500 μM EM20-25 (EM) for 1 h with or without a 3-h preincubation with IND (3 μM) and/or a 30-min preincubation with EIP (50 μM). Numbers are mean values of percentages (±SD) of four FACS analyses. (G) Model of CyP-D regulation by ERK2/GSK-3. Red denotes the final sensitizing effect on the PTP (i.e., an increased probability of pore opening). PP, protein phosphatase; PPi, protein phosphatase inhibitor.
Similar articles
- Induction of the permeability transition pore in cells depleted of mitochondrial DNA.
Masgras I, Rasola A, Bernardi P. Masgras I, et al. Biochim Biophys Acta. 2012 Oct;1817(10):1860-6. doi: 10.1016/j.bbabio.2012.02.022. Epub 2012 Feb 28. Biochim Biophys Acta. 2012. PMID: 22402226 - Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3α/β and Bax, leading to permeability transition pore opening and tumor cell death.
Chiara F, Gambalunga A, Sciacovelli M, Nicolli A, Ronconi L, Fregona D, Bernardi P, Rasola A, Trevisan A. Chiara F, et al. Cell Death Dis. 2012 Dec 13;3(12):e444. doi: 10.1038/cddis.2012.184. Cell Death Dis. 2012. PMID: 23235461 Free PMC article. - SERPINB3 protects from oxidative damage by chemotherapeutics through inhibition of mitochondrial respiratory complex I.
Ciscato F, Sciacovelli M, Villano G, Turato C, Bernardi P, Rasola A, Pontisso P. Ciscato F, et al. Oncotarget. 2014 May 15;5(9):2418-27. doi: 10.18632/oncotarget.1411. Oncotarget. 2014. PMID: 24810714 Free PMC article. - Signal transduction to the permeability transition pore.
Rasola A, Sciacovelli M, Pantic B, Bernardi P. Rasola A, et al. FEBS Lett. 2010 May 17;584(10):1989-96. doi: 10.1016/j.febslet.2010.02.022. Epub 2010 Feb 11. FEBS Lett. 2010. PMID: 20153328 Free PMC article. Review. - Genetic dissection of the permeability transition pore.
Forte M, Bernardi P. Forte M, et al. J Bioenerg Biomembr. 2005 Jun;37(3):121-8. doi: 10.1007/s10863-005-6565-9. J Bioenerg Biomembr. 2005. PMID: 16167169 Review.
Cited by
- Mitochondrial Energy Metabolism and Thyroid Cancers.
Lee J, Chang JY, Kang YE, Yi S, Lee MH, Joung KH, Kim KS, Shong M. Lee J, et al. Endocrinol Metab (Seoul). 2015 Jun;30(2):117-23. doi: 10.3803/EnM.2015.30.2.117. Endocrinol Metab (Seoul). 2015. PMID: 26194071 Free PMC article. Review. - The Dual Function of Reactive Oxygen/Nitrogen Species in Bioenergetics and Cell Death: The Role of ATP Synthase.
Kaludercic N, Giorgio V. Kaludercic N, et al. Oxid Med Cell Longev. 2016;2016:3869610. doi: 10.1155/2016/3869610. Epub 2016 Mar 10. Oxid Med Cell Longev. 2016. PMID: 27034734 Free PMC article. Review. - The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology.
Bernardi P, Rasola A, Forte M, Lippe G. Bernardi P, et al. Physiol Rev. 2015 Oct;95(4):1111-55. doi: 10.1152/physrev.00001.2015. Physiol Rev. 2015. PMID: 26269524 Free PMC article. Review. - T-cell death following immune activation is mediated by mitochondria-localized SARM.
Panneerselvam P, Singh LP, Selvarajan V, Chng WJ, Ng SB, Tan NS, Ho B, Chen J, Ding JL. Panneerselvam P, et al. Cell Death Differ. 2013 Mar;20(3):478-89. doi: 10.1038/cdd.2012.144. Epub 2012 Nov 23. Cell Death Differ. 2013. PMID: 23175186 Free PMC article. - Location Bias as Emerging Paradigm in GPCR Biology and Drug Discovery.
Mohammad Nezhady MA, Rivera JC, Chemtob S. Mohammad Nezhady MA, et al. iScience. 2020 Oct 7;23(10):101643. doi: 10.1016/j.isci.2020.101643. eCollection 2020 Oct 23. iScience. 2020. PMID: 33103080 Free PMC article. Review.
References
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. - PubMed
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. - PubMed
- Gioeli D. Signal transduction in prostate cancer progression. Clin Sci (Lond) 2005;108:293–308. - PubMed
- Dhanasekaran DN, Johnson GL. MAPKs: Function, regulation, role in cancer and therapeutic targeting. Oncogene. 2007;26:3097–3099. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous