AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis - PubMed (original) (raw)
doi: 10.1038/ng.519. Epub 2010 Jan 17.
Gianluca Caridi, Vanda S Lopes, Francesco Brancati, Andreas Kispert, Madeline A Lancaster, Andrew M Schlossman, Edgar A Otto, Michael Leitges, Hermann-Josef Gröne, Irma Lopez, Harini V Gudiseva, John F O'Toole, Elena Vallespin, Radha Ayyagari, Carmen Ayuso, Frans P M Cremers, Anneke I den Hollander, Robert K Koenekoop, Bruno Dallapiccola, Gian Marco Ghiggeri, Friedhelm Hildebrandt, Enza Maria Valente, David S Williams, Joseph G Gleeson
Affiliations
- PMID: 20081859
- PMCID: PMC2884967
- DOI: 10.1038/ng.519
AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis
Carrie M Louie et al. Nat Genet. 2010 Feb.
Abstract
Degeneration of photoreceptors is a common feature of ciliopathies, owing to the importance of the specialized ciliary structure of these cells. Mutations in AHI1, which encodes a cilium-localized protein, have been shown to cause a form of Joubert syndrome that is highly penetrant for retinal degeneration. We show that Ahi1-null mice fail to form retinal outer segments and have abnormal distribution of opsin throughout their photoreceptors. Apoptotic cell death of photoreceptors occurs rapidly between 2 and 4 weeks of age in these mice and is significantly (P = 0.00175 and 0.00613) delayed by a reduced dosage of opsin. This phenotype also shows dosage-sensitive genetic interactions with Nphp1, another ciliopathy-related gene. Although it is not a primary cause of retinal blindness in humans, we show that an allele of AHI1 is associated with a more than sevenfold increase in relative risk of retinal degeneration within a cohort of individuals with the hereditary kidney disease nephronophthisis. Our data support context-specific roles for AHI1 as a contributor to retinopathy and show that AHI1 may explain a proportion of the variability in retinal phenotypes observed in nephronophthisis.
Figures
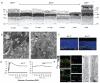
Figure 1
Degeneration of photoreceptor cells following failed outer segment development in _Ahi1_−/− mouse retina. (a) Semi-thin sections of retina stained with toluidine blue from P10 to adult (10 wks) showing acute loss of the outer nuclear layer (ONL) between P21 and P30. Scale=20μm (b) Transmission electron microscopy from P10. Outer segments (OS, brackets) are present in Ahi1+/−, but not in _Ahi1_−/−. Connecting cilia (CC, arrowheads) present in both. Scale=0.5 μm (c) Photoreceptor cell death is evident before three weeks of age, indicated by activated caspase-3 immunofluorescence (green) from 20μm cryosections. Nuclei are stained with Hoescht 33342 (blue). Shown are representative images from P19 mice, scale=20μm. (d) Full field dark-adapted electroretinograms (ERGs) from P19 Ahi1flox/−; Nes-Cre+ (_Ahi1_Nestin cKO) and Ahi1 heterozygous control mice. Shown are representative waveforms from _n_=3-4 mice/genotype. (e) Endogenous Ahi1 localization (red) to base of photoreceptor connecting cilium (acetylated-tubulin: green) in Ahi1+/− section is absent in _Ahi1_−/− cilium. Ahi1 distribution overlaps with centrin-2 (green) in GFP-Cetn-2 transgenic mouse retina. Arrowheads indicating example GFP-centrin2 labeled connecting cilia, 10μm cryosections, scale=5μm. Ahi1 immunoelectron microscopy from P10 retina, showing particles at the basal body (BB) and along the cilium (CC), scale=0.25μm
Figure 2
Opsin accumulation in Ahi1−/− photoreceptors (a) Opsin immunofluorescence (green) at P10 in Ahi1+/− and _Ahi1_−/− retina from cryosections. Nuclei are stained with Hoescht 33342. Scale=20μm (b) Opsin immunoEM and quantification of immunogold labeling from ultrathin sections of P10 Ahi1+/− and _Ahi1_−/− retina, _n_=20-23 photoreceptor connecting cilia/genotype. Data are expressed as number of gold particles per μm2 area within the inner segment (IS, defined as within inner segment and at least 30nm away from PM, _P_=8.0E-06), and as number of particles per μm length of inner segment membranes (PM, dashed lines, _P_=2.2E-07). Black arrowheads indicate examples of abnormally localized opsin, CC=connecting cilium, scale=0.5μm, error bars represent s.e.m.
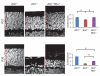
Figure 3
Opsin contributes to cell death in _Ahi1_−/− mice. 10μm cryosections from P21 and P30 retina showing delay of cell loss associated with reduced opsin dosage (Ahi1_−/−_Rho+/−), measured as average number of cells/column (nuclei were stained with Hoescht 33342 and counts expressed as average of three counts across each section). Asterisk in top panel denotes significant difference from both control and rescue, _n_=3-7, _P_=0.00175 (P21) and 0.00613 (P30), scale=10μm, error bars represent s.e.m.
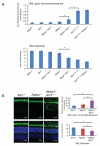
Figure 4
Genetic interaction of Ahi1 with Nphp1. (a) Increased cell loss and redistribution of opsin with increased load of deleterious Ahi1 and Nphp1 mutations in mouse retina. Quantification of opsin immunofluorescence(green) from ONL (outer nuclear layer, normalized as ratio of ONL:apical region encompassing IS and OS), and quantification of outer nuclear layer (ONL) thickness, expressed as averaged number of nuclei/column (indicated by Hoescht 33342 staining) from 10μm cryosections from indicated genotypes at P21. (b) From part (a), Ahi1 null allele modifies the _Nphp1_−/− phenotype, with Nphp1_−/−_Ahi1+/− showing increased opsin accumulation and decreased thickness of the ONL versus _Nphp1_−/− and Ahi1+/− controls. Asterisk denotes significant difference from both Ahi1+/− and _Nphp1_−/−, _n_=6-7, P(ONL thickness)=0.00315 and 0.000106, respectively; and P(fluorescence)=0.0454 and 0.0141, respectively, scale=20μm, error bars represent s.e.m.
Similar articles
- Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1.
Westfall JE, Hoyt C, Liu Q, Hsiao YC, Pierce EA, Page-McCaw PS, Ferland RJ. Westfall JE, et al. J Neurosci. 2010 Jun 30;30(26):8759-68. doi: 10.1523/JNEUROSCI.5229-09.2010. J Neurosci. 2010. PMID: 20592197 Free PMC article. - AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome.
Parisi MA, Doherty D, Eckert ML, Shaw DW, Ozyurek H, Aysun S, Giray O, Al Swaid A, Al Shahwan S, Dohayan N, Bakhsh E, Indridason OS, Dobyns WB, Bennett CL, Chance PF, Glass IA. Parisi MA, et al. J Med Genet. 2006 Apr;43(4):334-9. doi: 10.1136/jmg.2005.036608. Epub 2005 Sep 9. J Med Genet. 2006. PMID: 16155189 Free PMC article. - Differential requirement of NPHP1 for compartmentalized protein localization during photoreceptor outer segment development and maintenance.
Datta P, Cribbs JT, Seo S. Datta P, et al. PLoS One. 2021 May 7;16(5):e0246358. doi: 10.1371/journal.pone.0246358. eCollection 2021. PLoS One. 2021. PMID: 33961633 Free PMC article. - Primary cilia biogenesis and associated retinal ciliopathies.
Chen HY, Kelley RA, Li T, Swaroop A. Chen HY, et al. Semin Cell Dev Biol. 2021 Feb;110:70-88. doi: 10.1016/j.semcdb.2020.07.013. Epub 2020 Jul 31. Semin Cell Dev Biol. 2021. PMID: 32747192 Free PMC article. Review. - Non-syndromic retinal ciliopathies: translating gene discovery into therapy.
Estrada-Cuzcano A, Roepman R, Cremers FP, den Hollander AI, Mans DA. Estrada-Cuzcano A, et al. Hum Mol Genet. 2012 Oct 15;21(R1):R111-24. doi: 10.1093/hmg/dds298. Epub 2012 Jul 26. Hum Mol Genet. 2012. PMID: 22843501 Review.
Cited by
- Polymorphic variation of RPGRIP1L and IQCB1 as modifiers of X-linked retinitis pigmentosa caused by mutations in RPGR.
Fahim AT, Bowne SJ, Sullivan LS, Webb KD, Williams JT, Wheaton DK, Birch DG, Daiger SP. Fahim AT, et al. Adv Exp Med Biol. 2012;723:313-20. doi: 10.1007/978-1-4614-0631-0_41. Adv Exp Med Biol. 2012. PMID: 22183348 Free PMC article. No abstract available. - Phenotypic spectrum and prevalence of INPP5E mutations in Joubert syndrome and related disorders.
Travaglini L, Brancati F, Silhavy J, Iannicelli M, Nickerson E, Elkhartoufi N, Scott E, Spencer E, Gabriel S, Thomas S, Ben-Zeev B, Bertini E, Boltshauser E, Chaouch M, Cilio MR, de Jong MM, Kayserili H, Ogur G, Poretti A, Signorini S, Uziel G, Zaki MS; International JSRD Study Group; Johnson C, Attié-Bitach T, Gleeson JG, Valente EM. Travaglini L, et al. Eur J Hum Genet. 2013 Oct;21(10):1074-8. doi: 10.1038/ejhg.2012.305. Epub 2013 Feb 6. Eur J Hum Genet. 2013. PMID: 23386033 Free PMC article. - Protein Kinase A in Human Retina: Differential Localization of Cβ, Cα, RIIα, and RIIβ in Photoreceptors Highlights Non-redundancy of Protein Kinase A Subunits.
Roa JN, Ma Y, Mikulski Z, Xu Q, Ilouz R, Taylor SS, Skowronska-Krawczyk D. Roa JN, et al. Front Mol Neurosci. 2021 Nov 18;14:782041. doi: 10.3389/fnmol.2021.782041. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34867193 Free PMC article. - DNAH6 and Its Interactions with PCD Genes in Heterotaxy and Primary Ciliary Dyskinesia.
Li Y, Yagi H, Onuoha EO, Damerla RR, Francis R, Furutani Y, Tariq M, King SM, Hendricks G, Cui C, Saydmohammed M, Lee DM, Zahid M, Sami I, Leatherbury L, Pazour GJ, Ware SM, Nakanishi T, Goldmuntz E, Tsang M, Lo CW. Li Y, et al. PLoS Genet. 2016 Feb 26;12(2):e1005821. doi: 10.1371/journal.pgen.1005821. eCollection 2016 Feb. PLoS Genet. 2016. PMID: 26918822 Free PMC article. - Ciliopathy-associated protein CEP290 modifies the severity of retinal degeneration due to loss of RPGR.
Rao KN, Zhang W, Li L, Ronquillo C, Baehr W, Khanna H. Rao KN, et al. Hum Mol Genet. 2016 May 15;25(10):2005-2012. doi: 10.1093/hmg/ddw075. Epub 2016 Mar 2. Hum Mol Genet. 2016. PMID: 26936822 Free PMC article.
References
- Valente EM, et al. AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann Neurol. 2006;59:527–34. - PubMed
- Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: Primary cilia in development and disease. Curr Top Dev Biol. 2008;84:249–310. - PubMed
- Ferland RJ, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY013408-05A2/EY/NEI NIH HHS/United States
- F31NS059281/NS/NINDS NIH HHS/United States
- P30NS047101/NS/NINDS NIH HHS/United States
- R01EY007042/EY/NEI NIH HHS/United States
- R01DK068306/DK/NIDDK NIH HHS/United States
- R01 EY007042/EY/NEI NIH HHS/United States
- R01 NS048453/NS/NINDS NIH HHS/United States
- R01 DK068306/DK/NIDDK NIH HHS/United States
- GGP08145/TI_/Telethon/Italy
- T32 GM008666/GM/NIGMS NIH HHS/United States
- F31 NS059281/NS/NINDS NIH HHS/United States
- R01 EY013408/EY/NEI NIH HHS/United States
- R01NS048453/NS/NINDS NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous