Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals - PubMed (original) (raw)
Review
Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals
Barbara Stecca et al. J Mol Cell Biol. 2010 Apr.
Abstract
A surprisingly large and unrelated number of human tumors depend on sustained HEDGEHOG-GLI (HH-GLI) signaling for growth. This includes cancers of the skin, brain, colon, lungs, prostate, blood and pancreas among others. The basis of such commonality is not obvious. HH-GLI signaling has also been shown to be active in and required for cancer stem cell survival and expansion in different cancer types, and its activity is essential not only for tumor growth but also for recurrence and metastatic growth, two key medical problems. Here we review recent data on the role of HH-GLI signaling in cancer focusing on the role of the GLI code, the regulated combinatorial and cooperative function of repressive and activating forms of all Gli transcription factors, as a signaling nexus that integrates not only HH signals but also those of multiple tumor suppressors and oncogenes. Recent data support the view that the context-dependent regulation of the GLI code by oncogenes and tumor suppressors constitutes a basis for the widespread involvement of GLI1 in human cancers, representing a perversion of its normal role in the control of stem cell lineages during normal development and homeostasis.
Figures
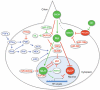
Figure 1
The GLI code. Different combinations of GLI activator (in green) and repressor forms (in red), with different potencies, are proposed to activate different sets of target genes that result in specific cellular fates and proliferation rates. The diagram illustrates how different (combinatorial and quantitative) GLI codes give different cellular outcomes.
Figure 2
Integration of oncogenic and tumor suppressor inputs by the GLI code in cancer. Upon inhibition of PTCH1 function by HH ligands, the repression on SMOH is released, SMOH moves into the primary cilium and activates downstream signaling by stabilizing activating full-length GLI proteins (GLI1) and blocking the production of GLI repressors (GLI3R). The mammalian GLI code includes three proteins. Generally, GLI1 is an activator although it exists in N′ and C′ Δ deleted activator and repressor forms, respectively, GLI2 has activator and C′ Δ repressor functions and GLI3 is a weak activator and its C′ Δ form is a strong repressor. Components of the classical HH pathway are in filled circles, in red for inhibitors and in green for activators. Positive and negative regulators of HH-GLI signaling are in unfilled circles, in blue for the PGF-RTK-RAS-RAF-MEK, PI3K-AKT and JUN pathways, in green for activators and in red for repressors. The color of the arrow is dictated by the final effect on the GLI code: red arrow for a final repressive effect, green arrow for a final activating effect on the GLI code. See text for further details.
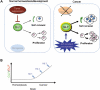
Figure 3
Regulation of the GLI code by oncogenic and tumor suppressor inputs in stem cells and cancer. During development, HH signaling tightly promotes the formation of labile GLI activators. The action of tumor suppressors further restrains GLI positive function, which is boosted by converging pathways and inputs such as those triggered by oncogenic PGF-RTK signals. (A and B) Mutations or epigenetic changes that lead to the loss of tumor suppressors (for example p53, PTEN) and activation of oncogenic pathways (for example RAS-RAF-MEK, PI3K-AKT) could unlock the normally restricted proliferative and self-renewing activities of GLI activators leading to an abnormal expansion of cancer stem cells (Ruiz i Altaba et al., 2007).
Similar articles
- Hedgehog signaling and the Gli code in stem cells, cancer, and metastases.
Ruiz i Altaba A. Ruiz i Altaba A. Sci Signal. 2011 Nov 22;4(200):pt9. doi: 10.1126/scisignal.2002540. Sci Signal. 2011. PMID: 22114144 - Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion.
Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Varnat F, et al. EMBO Mol Med. 2009 Sep;1(6-7):338-51. doi: 10.1002/emmm.200900039. EMBO Mol Med. 2009. PMID: 20049737 Free PMC article. - Cooperative integration between HEDGEHOG-GLI signalling and other oncogenic pathways: implications for cancer therapy.
Pandolfi S, Stecca B. Pandolfi S, et al. Expert Rev Mol Med. 2015 Feb 9;17:e5. doi: 10.1017/erm.2015.3. Expert Rev Mol Med. 2015. PMID: 25660620 Free PMC article. Review. - Context-dependent signal integration by the GLI code: the oncogenic load, pathways, modifiers and implications for cancer therapy.
Aberger F, Ruiz i Altaba A. Aberger F, et al. Semin Cell Dev Biol. 2014 Sep;33(100):93-104. doi: 10.1016/j.semcdb.2014.05.003. Epub 2014 May 19. Semin Cell Dev Biol. 2014. PMID: 24852887 Free PMC article. Review. - Therapeutic inhibition of Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell targets?
Ruiz i Altaba A. Ruiz i Altaba A. Cancer Cell. 2008 Oct 7;14(4):281-3. doi: 10.1016/j.ccr.2008.09.007. Cancer Cell. 2008. PMID: 18835029
Cited by
- Tamoxifen Resistance: Emerging Molecular Targets.
Rondón-Lagos M, Villegas VE, Rangel N, Sánchez MC, Zaphiropoulos PG. Rondón-Lagos M, et al. Int J Mol Sci. 2016 Aug 19;17(8):1357. doi: 10.3390/ijms17081357. Int J Mol Sci. 2016. PMID: 27548161 Free PMC article. Review. - The Hedgehog signalling pathway in breast development, carcinogenesis and cancer therapy.
Hui M, Cazet A, Nair R, Watkins DN, O'Toole SA, Swarbrick A. Hui M, et al. Breast Cancer Res. 2013 Mar 28;15(2):203. doi: 10.1186/bcr3401. Breast Cancer Res. 2013. PMID: 23547970 Free PMC article. Review. - Tumor microenvironment pathways: Cross regulation in breast cancer metastasis.
Malla RR, Kiran P. Malla RR, et al. Genes Dis. 2020 Dec 1;9(2):310-324. doi: 10.1016/j.gendis.2020.11.015. eCollection 2022 Mar. Genes Dis. 2020. PMID: 35224148 Free PMC article. Review. - Hedgehog signaling regulates telomerase reverse transcriptase in human cancer cells.
Mazumdar T, Sandhu R, Qadan M, DeVecchio J, Magloire V, Agyeman A, Li B, Houghton JA. Mazumdar T, et al. PLoS One. 2013 Sep 25;8(9):e75253. doi: 10.1371/journal.pone.0075253. eCollection 2013. PLoS One. 2013. PMID: 24086482 Free PMC article. - Role and inhibition of GLI1 protein in cancer.
Mastrangelo E, Milani M. Mastrangelo E, et al. Lung Cancer (Auckl). 2018 Mar 27;9:35-43. doi: 10.2147/LCTT.S124483. eCollection 2018. Lung Cancer (Auckl). 2018. PMID: 29628779 Free PMC article. Review.
References
- Ahn S., Joyner A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources