Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells - PubMed (original) (raw)
doi: 10.2337/db09-0498. Epub 2010 Jan 19.
Antonello Pileggi, Elsa Laughlin, Gloria Allende, Ainhoa Martin-Pagola, R Damaris Molano, Stavros Diamantopoulos, Nathan Standifer, Kelly Geubtner, Ben A Falk, Hirohito Ichii, Hidenori Takahashi, Isaac Snowhite, Zhibin Chen, Armando Mendez, Linda Chen, Junichiro Sageshima, Phillip Ruiz, Gaetano Ciancio, Camillo Ricordi, Helena Reijonen, Gerald T Nepom, George W Burke 3rd, Alberto Pugliese
Affiliations
- PMID: 20086230
- PMCID: PMC2844842
- DOI: 10.2337/db09-0498
Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells
Francesco Vendrame et al. Diabetes. 2010 Apr.
Abstract
Objective: To investigate if recurrent autoimmunity explained hyperglycemia and C-peptide loss in three immunosuppressed simultaneous pancreas-kidney (SPK) transplant recipients.
Research design and methods: We monitored autoantibodies and autoreactive T-cells (using tetramers) and performed biopsy. The function of autoreactive T-cells was studied with in vitro and in vivo assays.
Results: Autoantibodies were present pretransplant and persisted on follow-up in one patient. They appeared years after transplantation but before the development of hyperglycemia in the remaining patients. Pancreas transplant biopsies were taken within approximately 1 year from hyperglycemia recurrence and revealed beta-cell loss and insulitis. We studied autoreactive T-cells from the time of biopsy and repeatedly demonstrated their presence on further follow-up, together with autoantibodies. Treatment with T-cell-directed therapies (thymoglobulin and daclizumab, all patients), alone or with the addition of B-cell-directed therapy (rituximab, two patients), nonspecifically depleted T-cells and was associated with C-peptide secretion for >1 year. Autoreactive T-cells with the same autoantigen specificity and conserved T-cell receptor later reappeared with further C-peptide loss over the next 2 years. Purified autoreactive CD4 T-cells from two patients were cotransplanted with HLA-mismatched human islets into immunodeficient mice. Grafts showed beta-cell loss in mice receiving autoreactive T-cells but not control T-cells.
Conclusions: We demonstrate the cardinal features of recurrent autoimmunity in three such patients, including the reappearance of CD4 T-cells capable of mediating beta-cell destruction. Markers of autoimmunity can help diagnose this underappreciated cause of graft loss. Immune monitoring during therapy showed that autoimmunity was not resolved by the immunosuppressive agents used.
Figures
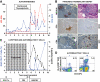
FIG. 1.
Clinical course, autoimmunity assessment, and biopsy in patient 1. Patient 1 was a 41-year-old Caucasian male [HLA A2/A3, B57/B60, DR4 (DRB1*0405)/DR6] who developed type 1 diabetes at age 7 years. He received an SPK transplant from an HLA A2/A30, B41/B60, DR4/DRX donor at age 32 years. The transplant reversed diabetes, but the patient returned to insulin dependence 5 years later, while kidney and exocrine pancreas allografts had normal function. A: Autoantibody levels before transplant and on follow-up. The patient had GAD and IA-2 autoantibodies before transplantation, which persisted despite immunosuppression, and titers increased on follow-up. Color-matched, horizontal lines represent the cutoff level for each autoantibody. For all autoantibodies, a value >1 denotes a positive result. B: Pancreas transplant biopsy stained as labeled, obtained ∼6 months after the recurrence of hyperglycemia. Insulitis and β-cell loss are shown. C: Serum C-peptide levels and % of GAD tetramer–positive T-cells in the CD4 T-cell population from the time of hyperglycemia recurrence. C-peptide was still detectable at diagnosis, confirming the function of residual β-cells observed at biopsy. Autoreactive T-cells were detected at the time of biopsy, ∼6 months after the recurrence of hyperglycemia on two samples, and again at several time points ∼1 year after treatment. The horizontal blue line represents the cutoff of the tetramer assay (0.25%). D: Flow cytometry plots demonstrating GAD-autoreactive CD4 T-cells. The numbers above the plots identify the same sample in C. Tetramer staining with irrelevant peptide was <0.1% (not shown). DM, diabetes; Tx, treatment. (A high-quality digital representation of this figure is available in the online issue.)
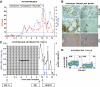
FIG. 2.
Clinical course, autoimmunity assessment, and biopsy in patient 2. Patient 2 is a Caucasian female (HLA A2/A24, B44/B56, DR5/DR9) who developed type 1 diabetes at age 8 years. She received an SPK transplant from an HLA A2/A3, B7/B14, DR7/DR9 donor at age 30 years. Her pancreas transplant successfully reversed diabetes. After approximately 9 years, the patient developed hyperglycemia requiring insulin therapy, while the function of the kidney and exocrine pancreas allografts remained unchanged. A: Autoantibody levels before transplant and on follow-up. The patient converted to GAD and IA-2 autoantibody positivity 6 years after transplantation. Hyperglycemia ensued 3.5 years after autoantibody conversion. B: Pancreas transplant biopsy stained as labeled, obtained ∼7 months after the recurrence of hyperglycemia. There was evidence for insulitis and β-cell loss. C: C-peptide levels from the time of hyperglycemia recurrence and % of IGRP tetramer–positive T-cells in the CD8 T-cell population. The horizontal blue line represents the cutoff of the T-cell assay (0.1%). Percentage of cells plotted is the specific staining value shown in D minus the background staining with control peptide. Circulating CD8 T-cells reacting against IGRP were found in a sample obtained at the time of biopsy and again ∼1 year after treatment. D: Flow cytometry plots showing IGRP-specific autoreactive CD8 T-cells. Staining with tetramers loaded with a control peptide yielded 0.1% background staining levels, gating on PBMC (not shown). The numbers above the plots identify the IGRP T-cell measurements in C, thus corresponding to the samples measured closest to the onset of hyperglycemia and over 1 year after treatment. DM, diabetes; Tx, treatment. (A high-quality digital representation of this figure is available in the online issue.)
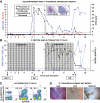
FIG. 3.
Clinical course, autoimmunity assessment, and biopsy in patient 3. Patient 3 is a 38-year-old Caucasian male [HLA A26/A30, B38/B58, DR3/DR4 (DRB1*0402)] who developed type 1 diabetes at age 12 years. He received an SPK transplant from an HLA-A23/A33, B7/B52, DR2/DR10 donor at age 27 years. The pancreas transplant successfully reversed diabetes. Five years later, the patient developed hyperglycemia requiring insulin therapy with unchanged function of the kidney and exocrine pancreas allografts. A: Autoantibody levels before transplant and on follow-up. Color-matched blue and black horizontal lines represent cutoffs for GAD/IA-2 and ZnT8 autoantibodies, respectively. The patient had been autoantibody negative before transplant and for almost 5 years on follow-up, but converted to GAD and ZnT8 autoantibody positivity about 3 months before the recurrence of hyperglycemia. At the time, there was a sharp rise in ZnT8 autoantibodies, shortly thereafter followed by a similar rise in GAD autoantibody levels, peaking at levels that were 40-fold and 10-fold higher than the upper limit of normal, respectively. Inset: Hormone stains in the first pancreas transplant biopsy obtained at retransplantation demonstrate β-cell loss. B: Serum C-peptide levels and % of GAD tetramer–positive T-cells in the CD4 T-cell population from the time of hyperglycemia recurrence. Patient 3 had no residual C-peptide secretion in the fasting state and no response to a Sustacal meal test (not shown) at the onset of hyperglycemia. C-peptide secretion was restored by retransplantation but was lost again after rejection of the second pancreas transplant. GAD-specific autoreactive CD4 T-cells were first studied in the sample obtained before the immunosuppression required for the second transplant. Autoreactive T-cells became undetectable after immunosuppression, but eventually rebounded and were detected on multiple occasions. The horizontal blue line represents the cutoff of the tetramer assay (0.25%). C: Flow cytometry plots demonstrating strong responses of GAD autoreactive, CD4 T-cells. Numbers above the plots correspond to those in B. Tetramer staining with irrelevant peptide was <0.1% (not shown). D: Biopsy of the second pancreas graft showing rejection. CD4 infiltrates are seen near residual insulin-stained areas. DM, diabetes; Tx, treatment. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 4.
In vivo assessment of the autoreactive potential of GAD-autoreactive CD4 T-cells from patient 1. A peripheral blood sample (sample no. 10, Fig. 1_C_ and D) from patient 1 yielded a very strong CD4 T-cell response to GAD; ∼7% of the CD4 T-cells were GAD autoreactive after in vitro stimulation and stained specifically with the DRB1*0405-GAD 555–567 tetramer (A). Approximately 15,000 tetramer-positive, GAD-autoreactive CD4 T-cells were purified by fluorescence-activated cell sorting and cotransplanted with human islets (1,300 islet equivalents), freshly isolated from an unrelated, deceased donor [HLA-A30, A33, B42, B70, DR8, DR17(3)], under the kidney capsule of a nondiabetic immunodeficient mouse. Control mice received islets with 15,000 CD4 T-cells from the same patient, which were stimulated with the HA control peptide and sorted after staining with a DRB1*0405-HA tetramer (B) or islets alone (C). H&E and insulin and glucagon stains representing the same areas of the graft reveal damaged islets and loss of insulin staining in the graft that received GAD-specific CD4 T-cells (A). Normal graft morphology and hormone staining patterns are seen in control mice receiving HA-specific CD4 T-cells (B) or islets alone (C). (A high-quality digital representation of this figure is available in the online issue.)
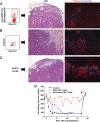
FIG. 5.
In vivo assessment of the autoreactive potential of GAD-autoreactive CD4 T-cells from patient 3. Immunodeficient mice with streptozotocin-induced diabetes were transplanted with human islets (2,000 islets equivalents) from an HLA-A1, A29, B8, B44, DR17(3)/DRX donor, which had been cultured for 24 h, and T-cells purified from blood obtained from patient 3 at 9.5 years of follow-up (sample no. 6, Fig. 3_B_). Specifically, we purified and cotransplanted islets with 6,000 tetramer-positive, GAD-autoreactive CD4 T-cells (6% of the CD4 T-cells, A). Control diabetic mice received islets and 30,000 CD4 T-cells after stimulation with the OspA control peptide (B) or islets alone (C). There was no response to this negative control peptide. Thus, this was a polyclonal T-cell population, which could have included alloreactive T-cells. CD25 staining confirmed that these T-cells had been activated in vitro (B). On metabolic follow-up (D), both control mice reversed their diabetes after islet transplantation, while the mouse that received the GAD-autoreactive CD4 T-cells remained hyperglycemic for the entire duration of the experiment. Control mice reverted to diabetes when the grafts were removed by nephrectomy (arrow) after ∼2 weeks. H&E stains from the islet-only control mouse showed mostly normal islets with some fibrosis in the surrounding tissue, as sometimes observed in these grafts (C). Insulin and glucagon stains in the same graft area revealed normal immunoreactivity for the islet-only control (C) and in the control mouse that received polyclonal, activated CD4 T-cells (B). However, H&E-stained sections revealed more fibrosis and inflammation, which could have been in part mediated by the polyclonal T-cells. H&E staining revealed severe islet destruction in the graft of the mouse transplanted with GAD-autoreactive CD4 T-cells, and hormone stains highlighted β-cell loss (A). (A high-quality digital representation of this figure is available in the online issue.)
Comment in
- The cardinal features of recurrent autoimmunity in simultaneous pancreas-kidney transplant recipients.
Hirshberg B. Hirshberg B. Curr Diab Rep. 2010 Oct;10(5):321-2. doi: 10.1007/s11892-010-0134-2. Curr Diab Rep. 2010. PMID: 20640940 No abstract available.
Similar articles
- Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation in the absence of GAD and IA-2 autoantibodies.
Assalino M, Genevay M, Morel P, Demuylder-Mischler S, Toso C, Berney T. Assalino M, et al. Am J Transplant. 2012 Feb;12(2):492-5. doi: 10.1111/j.1600-6143.2011.03844.x. Epub 2011 Dec 7. Am J Transplant. 2012. PMID: 22151900 - Lessons From Pancreas Transplantation in Type 1 Diabetes: Recurrence of Islet Autoimmunity.
Burke GW 3rd, Vendrame F, Virdi SK, Ciancio G, Chen L, Ruiz P, Messinger S, Reijonen HK, Pugliese A. Burke GW 3rd, et al. Curr Diab Rep. 2015 Dec;15(12):121. doi: 10.1007/s11892-015-0691-5. Curr Diab Rep. 2015. PMID: 26547222 Review. - Evidence of recurrent type I diabetes following HLA-mismatched pancreas transplantation.
Petruzzo P, Andreelli F, McGregor B, Lefrançois N, Dawahra M, Feitosa LC, Dubernard JM, Thivolet C, Martin X. Petruzzo P, et al. Diabetes Metab. 2000 May;26(3):215-8. Diabetes Metab. 2000. PMID: 10880896 - A case of recurrent type 1 diabetes mellitus with insulitis of transplanted pancreas in simultaneous pancreas-kidney transplantation from cardiac death donor.
Ishida-Oku M, Iwase M, Sugitani A, Masutani K, Kitada H, Tanaka M, Iida M. Ishida-Oku M, et al. Diabetologia. 2010 Feb;53(2):341-5. doi: 10.1007/s00125-009-1593-3. Epub 2009 Nov 13. Diabetologia. 2010. PMID: 19911164 - Recurrence of autoimmunity following pancreas transplantation.
Burke GW 3rd, Vendrame F, Pileggi A, Ciancio G, Reijonen H, Pugliese A. Burke GW 3rd, et al. Curr Diab Rep. 2011 Oct;11(5):413-9. doi: 10.1007/s11892-011-0206-y. Curr Diab Rep. 2011. PMID: 21660419 Free PMC article. Review.
Cited by
- Cutting edge of immune response and immunosuppressants in allogeneic and xenogeneic islet transplantation.
Yue L, Li J, Yao M, Song S, Zhang X, Wang Y. Yue L, et al. Front Immunol. 2024 Sep 13;15:1455691. doi: 10.3389/fimmu.2024.1455691. eCollection 2024. Front Immunol. 2024. PMID: 39346923 Free PMC article. Review. - Transplantation: platform to study recurrence of disease.
Burke GW, Mitrofanova A, Fontanella AM, Vendrame F, Ciancio G, Vianna RM, Roth D, Ruiz P, Abitbol CL, Chandar J, Merscher S, Pugliese A, Fornoni A. Burke GW, et al. Front Immunol. 2024 Mar 1;15:1354101. doi: 10.3389/fimmu.2024.1354101. eCollection 2024. Front Immunol. 2024. PMID: 38495894 Free PMC article. - Impact of GAD65 and IA2 autoantibodies on islet allograft survival.
Lemos JRN, Poggioli R, Ambut J, Bozkurt NC, Alvarez AM, Padilla N, Vendrame F, Ricordi C, Baidal DA, Alejandro R. Lemos JRN, et al. Front Clin Diabetes Healthc. 2023 Nov 13;4:1269758. doi: 10.3389/fcdhc.2023.1269758. eCollection 2023. Front Clin Diabetes Healthc. 2023. PMID: 38028981 Free PMC article. - [Anesthesia for organ transplant patients].
Fiala A, Breitkopf R, Sinner B, Mathis S, Martini J. Fiala A, et al. Anaesthesiologie. 2023 Nov;72(11):773-783. doi: 10.1007/s00101-023-01332-x. Epub 2023 Oct 24. Anaesthesiologie. 2023. PMID: 37874343 Free PMC article. Review. German. - Immune surveillance and humoral immune responses in kidney transplantation - A look back at T follicular helper cells.
Subburayalu J. Subburayalu J. Front Immunol. 2023 Jul 12;14:1114842. doi: 10.3389/fimmu.2023.1114842. eCollection 2023. Front Immunol. 2023. PMID: 37503334 Free PMC article. Review.
References
- Eisenbarth GS: Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med 1986; 314: 1360– 1368 - PubMed
- Lieberman SM, DiLorenzo TP: A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens 2003; 62: 359– 377 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-50864/AI/NIAID NIH HHS/United States
- R01 DK070011/DK/NIDDK NIH HHS/United States
- U42 RR016603/RR/NCRR NIH HHS/United States
- M01RR16587/RR/NCRR NIH HHS/United States
- U19 AI050864/AI/NIAID NIH HHS/United States
- 3U42RR016603-06S1/RR/NCRR NIH HHS/United States
- 5R01-DK-070011/DK/NIDDK NIH HHS/United States
- M01 RR016587/RR/NCRR NIH HHS/United States
- DP3 DK085696/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials