APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis - PubMed (original) (raw)
Review
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis
Leslie Crews et al. Brain Struct Funct. 2010 Mar.
Abstract
Neurodegenerative disorders of the aging population affect over 5 million people in the US and Europe alone. The common feature is the progressive accumulation of misfolded proteins with the formation of toxic oligomers. Alzheimer's disease (AD) is characterized by cognitive impairment, progressive degeneration of neuronal populations in the neocortex and limbic system, and formation of amyloid plaques and neurofibrillary tangles. Amyloid-beta (Abeta) is the product of proteolysis of amyloid precursor protein (APP) by beta and gamma-secretase enzymes. The neurodegenerative process in AD initiates with axonal and synaptic damage and is associated with progressive accumulation of toxic Abeta oligomers in the intracellular and extracellular space. In addition, neurodegeneration in AD is associated with alterations in neurogenesis. Abeta accumulation is the consequence of an altered balance between protein synthesis, aggregation rate, and clearance. Identification of genetic mutations in APP associated with familial forms of AD and gene polymorphisms associated with the more common sporadic variants of AD has led to the development of transgenic (tg) and knock out rodents as well as viral vector driven models of AD. While APP tg murine models with mutations in the N- and C-terminal flanking regions of Abeta are characterized by increased Abeta production with plaque formation, mutations in the mid-segment of Abeta result in increased formation of oligomers, and mutations toward the C-terminus (E22Q) segment results in amyloid angiopathy. Similar to AD, in APP tg models bearing familial mutations, formation of Abeta oligomers results in defective plasticity in the perforant pathway, selective neuronal degeneration, and alterations in neurogenesis. Promising results have been obtained utilizing APP tg models of AD to develop therapies including the use of beta- and gamma-secretase inhibitors, immunization, and stimulating neurogenesis.
Figures
Fig. 1
Schematic diagram of APP processing and accumulation of toxic Aβ species. β- and γ-secretase cleavage of APP results in the production of Aβ1–40 and Aβ1–42, which accumulates into neurotoxic oligomers
Fig. 2
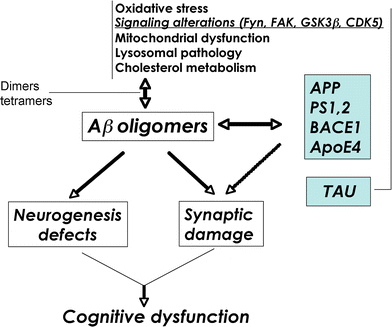
Schematic diagram of factors contributing to Aβ oligomerization. Defective cellular processes can lead to the accumulation of Aβ dimers, trimers, and oligomers, which in turn contribute to neurogenesis defects and synaptic damage
Fig. 3
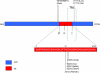
Diagram showing common mutations in the APP gene that are utilized in the generation of animal models of AD. Mutations in the N- and C-terminal domains of APP result in the accumulation of intracellular and/or extracellular Aβ species, while mutations in the Aβ region lead to the development of amyloid angiopathy. Swe Swedish mutation, Lon London mutation, Ind Indiana mutation, Arc Arctic mutation, TM transmembrane domain
Fig. 4
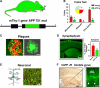
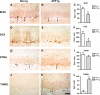
Characterization of cognitive and neuropathological alterations in the brains of mThy1-hAPP tg mice. a Structure of mutant hAPP transgene under the control of the mThy-1 promoter. b Memory portion of the water maze behavioral test where the platform was removed (Probe test) to evaluate the number of entrances into the target quadrant where the platform was previously located (# Entrances), the number of times the animal passed over the location, where the platform was (# Passes), and time spent (Time) swimming in the target quadrant where the platform was previously located. APP tg mice exhibited reduced performance compared to non-tg controls in all three measures of memory retention in this behavioral test. c Aβ-immunoreactive deposits in the cortex of an APP tg mouse. Scale bar 50 μm. d Reduced synaptophysin immunoreactivity in the brain of an APP tg mouse. Scale bar 0.2 mm. e Degeneration of the MAP2-immunoreactive dendritic arbor in the cortex of an APP tg mouse. Scale bar 10 μm. f hAPP immunoreactivity in the dentate gyrus (DG) of an APP tg mouse. Scale bar 1 mm (left panel), 20 μm (right panel). *p < 0.05 compared to non-tg controls by Student’s _t_-test (n = 4 mice per group)
Fig. 5
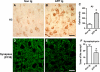
Increased intracellular pyroglutamate-Aβ immunoreactivity and synaptic deterioration in mThy1-hAPP tg mice. a–c Sections from the brains of non-tg and APP tg mice were immunolabeled with an antibody against pyroglutamate Aβ3–42 and developed with DAB. Increased intraneuronal immunoreactivity was detected in the cortex of APP tg mice compared to non-tg controls. Scale bar 20 μm. d–f Reduced synaptophysin (SY38) immunoreactivity in the neuropil of APP tg mice compared to non-tg controls. Scale bar 20 μm. *p < 0.05 compared to non-tg controls by Student’s _t_-test (n = 4 mice per group)
Fig. 6
Reduced markers of neurogenesis and increased apoptosis in the hippocampus of APP tg mice. a–c Reduced BrdU immunoreactivity in the hippocampal dentate gyrus of APP tg mice treated with BrdU compared to non-tg controls treated with BrdU. d–e Reduced doublecortin (DCX) immunoreactivity in the hippocampal dentate gyrus of APP tg mice compared to non-tg controls. g–i Reduced proliferating cell nuclear antigen (PCNA) immunoreactivity in the hippocampal dentate gyrus of APP tg mice compared to non-tg controls. j–l Increased TUNEL-positive cells in the hippocampal dentate gyrus of APP tg mice compared to non-tg controls. Scale bar 50 μm for all panels. *p < 0.05 compared to non-tg controls by Student’s _t_-test (n = 4 mice per group)
Fig. 7
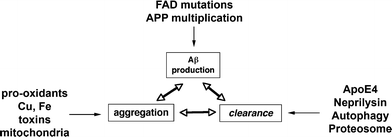
Schematic diagram showing several factors involved in the regulation of Aβ accumulation into oligomers, including production, aggregation, and clearance
Similar articles
- [Alzheimer disease: cellular and molecular aspects].
Octave JN. Octave JN. Bull Mem Acad R Med Belg. 2005;160(10-12):445-9; discussion 450-1. Bull Mem Acad R Med Belg. 2005. PMID: 16768248 French. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice.
Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Maier M, et al. J Neurosci. 2008 Jun 18;28(25):6333-41. doi: 10.1523/JNEUROSCI.0829-08.2008. J Neurosci. 2008. PMID: 18562603 Free PMC article. - BACE1 elevation is involved in amyloid plaque development in the triple transgenic model of Alzheimer's disease: differential Aβ antibody labeling of early-onset axon terminal pathology.
Cai Y, Zhang XM, Macklin LN, Cai H, Luo XG, Oddo S, Laferla FM, Struble RG, Rose GM, Patrylo PR, Yan XX. Cai Y, et al. Neurotox Res. 2012 Feb;21(2):160-74. doi: 10.1007/s12640-011-9256-9. Epub 2011 Jul 2. Neurotox Res. 2012. PMID: 21725719 Free PMC article. - [The beta-amyloid cascade hypothesis: a sequence of events leading to neurodegeneration in Alzheimer's disease].
Kowalska A. Kowalska A. Neurol Neurochir Pol. 2004 Sep-Oct;38(5):405-11. Neurol Neurochir Pol. 2004. PMID: 15565529 Review. Polish.
Cited by
- Effect of berberine on cognitive function and β-amyloid precursor protein in Alzheimer's disease models: a systematic review and meta-analysis.
Liu JY, Dai Y, He YX, Lin L. Liu JY, et al. Front Pharmacol. 2024 Jan 16;14:1301102. doi: 10.3389/fphar.2023.1301102. eCollection 2023. Front Pharmacol. 2024. PMID: 38293672 Free PMC article. - Abscisic Acid Supplementation Rescues High Fat Diet-Induced Alterations in Hippocampal Inflammation and IRSs Expression.
Ribes-Navarro A, Atef M, Sánchez-Sarasúa S, Beltrán-Bretones MT, Olucha-Bordonau F, Sánchez-Pérez AM. Ribes-Navarro A, et al. Mol Neurobiol. 2019 Jan;56(1):454-464. doi: 10.1007/s12035-018-1091-z. Epub 2018 May 2. Mol Neurobiol. 2019. PMID: 29721854 - Modeling human neurodegenerative diseases in transgenic systems.
Gama Sosa MA, De Gasperi R, Elder GA. Gama Sosa MA, et al. Hum Genet. 2012 Apr;131(4):535-63. doi: 10.1007/s00439-011-1119-1. Epub 2011 Dec 14. Hum Genet. 2012. PMID: 22167414 Review. - CaV1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-β plaques in an Alzheimer's disease mouse model.
Daschil N, Obermair GJ, Flucher BE, Stefanova N, Hutter-Paier B, Windisch M, Humpel C, Marksteiner J. Daschil N, et al. J Alzheimers Dis. 2013;37(2):439-51. doi: 10.3233/JAD-130560. J Alzheimers Dis. 2013. PMID: 23948887 Free PMC article. - Bioinformatics identification of modules of transcription factor binding sites in Alzheimer's disease-related genes by in silico promoter analysis and microarrays.
Augustin R, Lichtenthaler SF, Greeff M, Hansen J, Wurst W, Trümbach D. Augustin R, et al. Int J Alzheimers Dis. 2011 Apr 26;2011:154325. doi: 10.4061/2011/154325. Int J Alzheimers Dis. 2011. PMID: 21559189 Free PMC article.
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–2915. - PMC - PubMed
- Andra K, Abramowski D, Duke M, Probst A, Wiederholt K, Burki K, Goedert M, Sommer B, Staufenbiel M. Expression of APP in transgenic mice: a comparison of neuron-specific promoters. Neurobiol Aging. 1996;17:183–190. - PubMed
- Bertoli-Avella AM, Oostra BA, Heutink P. Chasing genes in Alzheimer’s and Parkinson’s disease. Hum Genet. 2004;114:413–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG11385/AG/NIA NIH HHS/United States
- R01 AG011385/AG/NIA NIH HHS/United States
- AG10435/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- NS44233/NS/NINDS NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 NS044233/NS/NINDS NIH HHS/United States
- R37 AG011385/AG/NIA NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P01 AG010435/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous