Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer's disease model mice - PubMed (original) (raw)
Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer's disease model mice
Latha Devi et al. Eur J Neurosci. 2010 Jan.
Abstract
Although transgenic mouse models of Alzheimer's disease (AD) recapitulate amyloid-beta (Abeta)-related pathologies and cognitive impairments, previous studies have mainly evaluated their hippocampus-dependent memory dysfunctions using behavioral tasks such as the water maze and fear conditioning. However, multiple memory systems become impaired in AD as the disease progresses and it is important to test whether other forms of memory are affected in AD models. This study was designed to use conditioned taste aversion (CTA) and contextual fear conditioning paradigms to compare the phenotypes of hippocampus-independent and -dependent memory functions, respectively, in 5XFAD amyloid precursor protein/presenilin-1 transgenic mice that harbor five familial AD mutations. Although both types of memory were significantly impaired in 5XFAD mice, the onset of CTA memory deficits ( approximately 9 months of age) was delayed compared with that of contextual memory deficits ( approximately 6 months of age). Furthermore, 5XFAD mice that were genetically engineered to have reduced levels of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) (BACE1(+/-).5XFAD) exhibited improved CTA memory, which was equivalent to the performance of wild-type controls. Importantly, elevated levels of cerebral beta-secretase-cleaved C-terminal fragment (C99) and Abeta peptides in 5XFAD mice were significantly reduced in BACE1(+/-).5XFAD mice. Furthermore, Abeta deposition in the insular cortex and basolateral amygdala, two brain regions that are critically involved in CTA performance, was also reduced in BACE1(+/-).5XFAD compared with 5XFAD mice. Our findings indicate that the CTA paradigm is useful for evaluating a hippocampus-independent form of memory defect in AD model mice, which is sensitive to rescue by partial reductions of the beta-secretase BACE1 and consequently of cerebral Abeta.
Figures
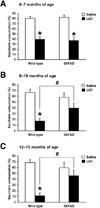
Fig. 1
Age-dependent impairments of conditioned taste aversion (CTA) in 5XFAD mice. (A–C) 5XFAD mice at 6–7 months (A), 9–10 months (B) and 12–15 months (C) of ages, and their respective wild-type littermate mice received LiCl (conditioned group) or saline (unconditioned group) after saccharin intake. While 5XFAD mice at 6–7 months of age exhibit significant reductions in percent saccharin consumption 1 day after conditioning compared to unconditioned controls (*P < 0.05 vs. saline), 5XFAD mice at 9–10 and 12–15 months of ages show preference for saccharin solution to levels not significantly different from that of unconditioned subjects and their CTA performance is significantly lower than that of wild-type controls (#P < 0.05). n = 8–18 mice per group. All data are presented as mean ± SEM.
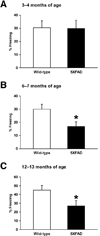
Fig. 2
Age-dependent impairments of contextual fear conditioning in 5XFAD mice. (A–C) 5XFAD mice at 3–4 months (A), 6–7 months (B) and 12–13 months (C) of ages, and their respective wild-type littermate mice were trained with two CS-US pairings for contextual fear conditioning. 5XFAD mice at 6–7 and 12–13 months of ages, but not at 3–4 months of age, show significantly lower levels of contextual freezing than wild-type controls (*P < 0.05) when tested 1 day after training. n = 11–23 mice per group. All data are presented as mean ± SEM.
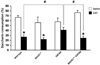
Fig. 3
Effects of heterozygous BACE1 deletion on impairments of conditioned taste aversion (CTA) in 5XFAD mice. Mice received LiCl (conditioned group) or saline (unconditioned group) after saccharin intake. Only 5XFAD mice show preference for saccharin solution to levels not significantly different from that of unconditioned subjects, whereas the other three groups exhibit significant reductions in percent saccharin consumption 1 day after conditioning compared to unconditioned controls (*P < 0.05 vs. saline). Note that 5XFAD mice at 9–10 months of ages show significantly lower levels of CTA to saccharin compared with wild-type littermate controls, while BACE1+/−·5XFAD are rescued completely back to wild-type levels of CTA performance (#P < 0.05 vs. 5XFAD). n = 10–25 mice per group. All data are presented as mean ± SEM.
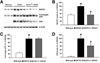
Fig. 4
Effects of heterozygous BACE1 deletion on levels of BACE1, full-length APP and C99 in brains of 5XFAD mice. (A) Immunoblot analysis of protein extracts from hemibrain homogenates of wild-type mice and 5XFAD mice with BACE1+/+ or BACE1+/− genotype at 9 months of age. (B–D) Intensities of immunoreactive bands on blots for BACE1 (B), full-length APP (C) and C99 (D) were quantified by phosphorimaging and expressed as percentage of 5XFAD levels. BACE1+/− genotype produces ~50% elimination of BACE1 expression and results in ~40% reductions in C99 without affecting APP overexpression in 5XFAD mice. Note that since BACE1 expression is significantly elevated in 5XFAD brains, levels of BACE1 that are equivalent to those of wild-type controls remain in BACE1+/−·5XFAD bigenic brains. *P < 0.05 (vs. wild-type controls), #P < 0.05 (vs. 5XFAD). n = 7–9 mice per group. All data are presented as mean ± SEM.
Fig. 5
Effects of heterozygous BACE1 deletion on Aβ levels in brains of 5XFAD mice. (A–B) Levels of total Aβ40 (A) and Aβ42 (B) were quantified by sandwich ELISAs of guanidine extracts of hemibrain samples and expressed as percentage of 9-month-old 5XFAD mice. Note that excessive levels of Aβ40 and Aβ42 are significantly reduced in BACE1+/−·5XFAD mice at 9 months of age compared with 5XFAD littermate controls (by ~65% and ~45%, respectively; #P < 0.05), while their residual Aβ levels are indistinguishable from those of 6-momth-old 5XFAD mice (N.S.: not significant). n = 7–9 mice per group. All data are presented as mean ± SEM.
Fig. 6
Effects of heterozygous BACE1 deletion on amyloid deposition in the insular cortex and basolateral amygdala of 5XFAD mice. (A, E) Diagrams illustrating the location of the insular cortex (A) and basolateral amygdala (E) (grey shaded areas). Schematic drawings of coronal sections were adapted from the mouse brain atlas of Franklin and Paxinos (2008). (B–D and F–H) Brain sections from wild-type control (B, F), 5XFAD (C, G) and BACE1+/−·5XFAD (D, H) mice at 9 months of age were immunostained with the 6E10 anti-Aβ antibody (n = 3 mice per group). Three different mice are presented for each of the 5XFAD (C1–C3, G1–G3) and BACE1+/−·5XFAD (D1–D3, H1–H3) genotypes. Shown are photomicrographs of the insular cortex (B–D) and basolateral amygdala (F–H) (areas within dashed ovals). Note that BACE1+/−·5XFAD brain sections exhibit lower levels of Aβ deposits in both brain regions than those of 5XFAD sections. Scale bar = 1 mm.
Similar articles
- Effects of BACE1 haploinsufficiency on APP processing and Aβ concentrations in male and female 5XFAD Alzheimer mice at different disease stages.
Devi L, Ohno M. Devi L, et al. Neuroscience. 2015 Oct 29;307:128-37. doi: 10.1016/j.neuroscience.2015.08.037. Epub 2015 Aug 24. Neuroscience. 2015. PMID: 26314636 Free PMC article. - Partial reduction of BACE1 improves synaptic plasticity, recent and remote memories in Alzheimer's disease transgenic mice.
Kimura R, Devi L, Ohno M. Kimura R, et al. J Neurochem. 2010 Apr;113(1):248-61. doi: 10.1111/j.1471-4159.2010.06608.x. Epub 2010 Jan 20. J Neurochem. 2010. PMID: 20089133 Free PMC article. - A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice.
Devi L, Ohno M. Devi L, et al. Mol Brain. 2015 Mar 25;8:19. doi: 10.1186/s13041-015-0110-5. Mol Brain. 2015. PMID: 25884928 Free PMC article. - BACE1: the beta-secretase enzyme in Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review. - Pharmacogenetic features of cathepsin B inhibitors that improve memory deficit and reduce beta-amyloid related to Alzheimer's disease.
Hook V, Hook G, Kindy M. Hook V, et al. Biol Chem. 2010 Aug;391(8):861-72. doi: 10.1515/BC.2010.110. Biol Chem. 2010. PMID: 20536395 Free PMC article. Review.
Cited by
- In vivo characterization of a bigenic fluorescent mouse model of Alzheimer's disease with neurodegeneration.
Crowe SE, Ellis-Davies GC. Crowe SE, et al. J Comp Neurol. 2013 Jul 1;521(10):2181-94. doi: 10.1002/cne.23306. J Comp Neurol. 2013. PMID: 23348594 Free PMC article. - Spines, plasticity, and cognition in Alzheimer's model mice.
Spires-Jones T, Knafo S. Spires-Jones T, et al. Neural Plast. 2012;2012:319836. doi: 10.1155/2012/319836. Epub 2011 Nov 28. Neural Plast. 2012. PMID: 22203915 Free PMC article. Review. - Effects of BACE1 haploinsufficiency on APP processing and Aβ concentrations in male and female 5XFAD Alzheimer mice at different disease stages.
Devi L, Ohno M. Devi L, et al. Neuroscience. 2015 Oct 29;307:128-37. doi: 10.1016/j.neuroscience.2015.08.037. Epub 2015 Aug 24. Neuroscience. 2015. PMID: 26314636 Free PMC article. - TrkB Receptor Agonist 7,8 Dihydroxyflavone is Protective Against the Inner Retinal Deficits Induced by Experimental Glaucoma.
Gupta V, Chitranshi N, Gupta V, You Y, Rajput R, Paulo JA, Mirzaei M, van den Buuse M, Graham SL. Gupta V, et al. Neuroscience. 2022 May 10;490:36-48. doi: 10.1016/j.neuroscience.2022.01.020. Epub 2022 Feb 23. Neuroscience. 2022. PMID: 35217121 Free PMC article.
References
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn. Mem. 2001;8:301–308. - PubMed
- Berger-Sweeney J, McPhie DL, Arters JA, Greenan J, Oster-Granite ML, Neve RL. Impairments in learning and memory accompanied by neurodegeneration in mice transgenic for the carboxyl-terminus of the amyloid precursor protein. Brain Res. Mol. Brain Res. 1999;66:150–162. - PubMed
- Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat. Rev. Neurosci. 2004;5:209–217. - PubMed
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 2001;4:233–234. - PubMed
- Carlesimo GA, Oscar-Berman M. Memory deficits in Alzheimer's patients: a comprehensive review. Neuropsychol. Rev. 1992;3:119–169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases