Beta-secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development - PubMed (original) (raw)
Beta-secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development
Xue-Mei Zhang et al. Eur J Neurosci. 2009 Dec.
Abstract
The presence of neuritic plaques is a pathological hallmark of Alzheimer's disease (AD). However, the origin of extracellular beta-amyloid peptide (Abeta) deposits and the process of plaque development remain poorly understood. The present study attempted to explore plaque pathogenesis by localizing beta-secretase-1 (BACE1) elevation relative to Abeta accumulation and synaptic/neuritic alterations in the forebrain, using transgenic mice harboring familial AD (FAD) mutations (5XFAD and 2XFAD) as models. In animals with fully developed plaque pathology, locally elevated BACE1 immunoreactivity (IR) coexisted with compact-like Abeta deposition, with BACE1 IR occurring selectively in dystrophic axons of various neuronal phenotypes or origins (GABAergic, glutamatergic, cholinergic or catecholaminergic). Prior to plaque onset, localized BACE1/Abeta IR occurred at swollen presynaptic terminals and fine axonal processes. These BACE1/Abeta-containing axonal elements appeared to undergo a continuing process of sprouting/swelling and dystrophy, during which extracellular Abeta IR emerged and accumulated in surrounding extracellular space. These data suggest that BACE1 elevation and associated Abeta overproduction inside the sprouting/dystrophic axonal terminals coincide with the onset and accumulation of extracellular amyloid deposition during the development of neuritic plaques in transgenic models of AD. Our findings appear to be in harmony with an early hypothesis that axonal pathogenesis plays a key or leading role in plaque formation.
Figures
Fig. 1
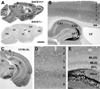
Immunohistochemical characterization of BACE1 in non-transgenic mouse brain using rabbit anti-BACE1α. Panel A shows selective BACE1 labeling in wild-type (BACE1+/+, top) but no signal in knockout (BACE1−/−, bottom) mouse brains following a batch processing of sagittal sections. The boxed area in A is enlarged as panel B. Panel C illustrates BACE1 labeling in a coronal section at the level of medial geniculate nucleus (MGN) from a 6 month-old C57BL/6L mouse, with two boxed areas enlarged as D and E. Note heavy BACE1 labeling in the olfactory bulb (OB) glomeruli (A) and hippocampal mossy fiber (mf) terminals (B, E). Diffuse and weak neuropil reactivity is present in the cortex (Ctx), amygdala and the remaining hippocampal areas (rather than mossy fiber terminals). Arab numbers indicate cortical layers. CBL: cerebellum, LV: lateral ventricle, St: striatum, Th: thalamus, VP: ventral pallidum, SC: superior colliculus, SNr: substantia nigra pars reticulata, WM: white matter, CA1-3: CA sectors of hippocampus, GCL: granule cell layer, ML(o) outer molecular layer, ML(i) inner molecular layer. Scale bar=2 mm in A, equal to 1 mm in C, 250 µm in B and 100 µm in D and E.
Fig. 2
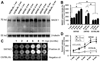
Biochemical analyses of age-related elevation of BACE1 protein levels (A, B), enzymatic activity of β-site amyloid precursor protein (APP) cleavage (C, D) and soluble Aβ levels (D). Values are expressed as mean±S.D. (%) normalized to indicated animal groups. Panel A shows a representative western analysis of cortical lysates from 5XFAD, 2XFAD and C57BL/6L control mice at indicated age points. BACE1 levels appear to increase with age in the transgenics relative to non-transgenic cohorts. Panel B summarizes data from three separate assays illustrating statistically significant (*) elevation of BACE1 protein levels in transgenics with age, and relative to non-transgenics. Panel C shows an example of BACE1 enzyme activity assay, illustrating increased fluorescent signals with age in 5XFAD samples relative to controls. Positive and negative assay controls are defined by incubations using company-supplied standard BACE1 enzyme and BACE1 inhibitor, respectively. Levels of soluble pan-Aβ species are determined by ELISA. Panel D summarizes results from 3 correlated enzyme activity and ELISA tests. BACE1 activity and Aβ levels increase in parallel in 5XFAD cortical extracts age-dependently, and are higher relative to non-transgenics counterparts.
Fig. 3
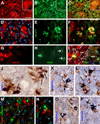
Selective BACE1 immunoreactivity (ir) in 5XFAD (D–K) and 2XFAD (L–Q) mouse brains around compact-like plaques. In batch-processed sections from a 12 month-old C57BL/6L mouse, BACE1 immunofluorescence appears as weak, background-like, reactivity in the cortex and hippocampus except for a heavy labeling at mossy fiber terminals (A–C). At low magnification, BACE1-ir occurs around virtually all plaque profiles visualized by monoclonal Aβ antibody 3D6 over the cortex in a 6 month-old 5XFAD (D–G) and a 12 month-old 2XFAD (L–O) mice. Shorter exposure (200 ms) for 3D6-ir displays compact plaques in star-fish appearance, with heaviest labeling localized to the plaque center surrounded by BACE1 labeled elements (H–J). With a slightly longer exposure (200 ms), 3D6-ir appears to extend beyond BACE1 labeled profiles around individual plaques but occur inside BACE1 labeled profiles (K, arrows). Panels P and Q show small perisomatic elements (indicated by arrows) labeled for BACE1 (P) and 3D6 (Q) together with bisbenzimide counterstain (blue), in addition to a large plaque on the top-right edge of the images (pointed by fat yellow arrows). These small BACE1/Aβ immunoreactive profiles will be further addressed in detail. Amyg: amygdala, DG: dentate gyrus, s.o.: stratum oriens, s.p.: stratum pyramidale; s.r.: stratum radiatum, s.l.m.: stratum lacunosum-moleculare. Scale bar=500 µm in A, equal to 1 mm in D and L, 250 µm in E–G, M–O; and 100 µm in B, C, H–K, P, Q.
Fig. 4
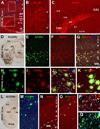
Localization of amyloidogenic proteins to axonal neurites around compact plaques in 5XFAD (8 month-old) mouse cortex and hippocampus. Panels A–D show virtually a complete colocalization of BACE1 with synaptophysin (SYN) in the dentate gyrus (A) and cortex (B–D). Panels E–H illustrate BACE1 colocalization with growth associated protein-43 (GAP43) in the hippocampal formation (E) and cortex (F–H). Some BACE1-contaning swollen terminals express low GAP-43 (arrowheads). BACE1 labeling does not colocalize with microtubule associated protein-2 (MAP2) (I–L). BACE1 labeled neurites also co-express APP (M–O) and possibly presenilin-1 (PS1) (P–R). Note that the monoclonal antibody 6E10 labels extracellular Aβ deposits but also neuronal somata (P). Scale bar=250 µm in A applying to E, equal to 100 µm in I, 50 µm for remaining panels.
Fig. 5
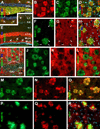
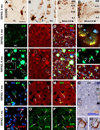
BACE1 localization to axonal dystrophic neurites in 5XFAD mouse forebrain. Subsets of BACE1 labeled dystrophic neurites coexpress glutamic acid decarboxylase-67 (GAD67) (A–C), vesicular glutamate transporter-1 (VGLUT1) (D-F), choline acetyltransferase (ChAT) (G–I). Panel J illustrates coexistence of BACE1-ir and β-nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) reactivity in dystrophic neurites (arrows) following a shorter histochemical reaction (15 minutes). Panels K and L show examples of dystrophic axons deriving from NADPH-d positive neurons (45 minutes reaction). Dystrophic neurites around the same plaque may express different neurotransmitter markers as indicated (M–P). NBM: nucleus basalis of Meynert. Arrows in (H, Q) point to cholinergic neuronal somata. Scale bar=50 µm in A, applying to B–I, M, N; equal to 25 µm in K, L, O, P; and 12.5 µm in J.
Fig. 6
Occurrence of BACE1 and Aβ labelings at perisomatic axon terminals in 5XFAD mice before and after plaque onset at indicated ages. In a 2 month-old animal (A–D), 3D6-ir occurs over and/or around the perikarya of large layer V (A, B) and CA1 (C) pyramidal neurons as small granules, with varying densities from cell to cell. Small clusters of swollen neurites appear in layer V commonly next to pyramidal perikarya and are associated with local extracellular Aβ reactivity (A, B, arrows). Note that 3D6 and 6E10 both label extracellular Aβ deposits, whereas 6E10-ir, but not 3D6, also occurs in the cytoplasm of pyramidal neurons (D). In a 1 month-old mouse, BACE1 and 3D6 colocalization occurs selectively around a subset of layer V pyramidal neurons (E–G), with labeled elements appearing granule-like at high magnification (G1). Panels H–J2 show a trend of evolution of BACE1/3D6 labeled perisomatic elements into "mini-plaques" consisted of small clusters of swollen neurites surrounded by local extracellular Aβ reactivity in 6 month-old mouse cortex. Several established compact plaques are present in the same field, which exhibit very bright 3D6 immunofluorescence. Panels K to M show colocalization of perisomatic 3D6-ir with synaptophysin (rabbit anti-SYN) around large layer V perikarya at 1 month of age. Similarly, BACE1 and synaptophysin (mouse anti-SYN) labelings colocalize at these perisomatic terminals in pre-plaque cortex (N–P). 3D6-ir also overlap locally at fine NADPH-d processes around or away from pyramidal perikarya (Q–Q2). OC: occipital cortex; TC: temporal cortex; FC: frontal cortex; Sub: subiculum. Scale bar=50 µm in A applying to K–M, J1–2 and N–O; equal to 200 µm for C, D; 100 µm for E–G, H–J and Q; and 12.5 µm for G1, Q1–2.
Fig. 7
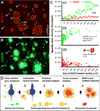
Densitometry and schematic drawing depicting a hypothesis that the onset and evolution of neuritic plaque may relate to a progressive axonal pathology inherent with intra-axonal Aβ genesis and local extracellular (extra-axonal) deposition. Panels A and B illustrate a method quantifying the areal sizes of, and BACE1/3D6-ir associated with, individual clusters of swollen/dystrophic neurites. Yellow cycles show examples for sampling the same set of profiles in BACE1 and 3D6 immunofluorescent images. Panel C1 plots data from 3 different microscopic fields (in the same section from a 6 month-old 5XFAD animal) showing relative specific densities of BACE1/3D6-ir among 42 clusters. BACE1-ir appears comparable among these clusters, whereas 3D6-ir appears to increase as clusters become >50–100 µm2. Panels C2 and C3 show trends of 3D6/BACE1-ir as a function of cluster size among ~150 profiles with areal sizes ≥ 50 µm2 (data were from 15 high power fields from 3 animals, with 489 clusters quantified in total). 3D6-ir increases rapidly as the clusters enlarge from 50–1000 µm2 (C2), whereas BACE1-ir maintains relatively stable (with a trend of decline in clusters larger than 1000 µm2) (C3). Based on the above data and our anatomical studies, a hypothesis for neuritic plaque onset and evolution is illustrated schematically (D). Thus, Aβ rise at presynaptic terminals and/or axonal processes, mediated by BACE1 elevation at least in part, may consist of the first step of plaque-forming process. Axonal pathology manifests as continuous swelling and sprouting, resulting in the formation of dystrophic neurites. Meanwhile, the diseased axons produce and release Aβ into surrounding extracellular space. At certain stage, Aβ deposits begin to accumulate at the center inside the growing neuritic cluster (core-formation) because secreted Aβ from all or the majority of these neurites can reach this site (an overlapping zone). Cored Aβ deposits may stimulate or force neuritic outgrowth preferentially towards the periphery, resulting in a rosette-like neuritic distribution pattern. Multi-cored plaques may form as axonal pathology and amyloidogenesis continue, or as neighboring plaques merge. Arab numbers and arrows in panels A, B and C point to representative profiles at artificially-defined corresponding stages of plaque development, including those potentially at transitional states (e.g., profiles marked with "3/4" and "4/5" in A, B).
Similar articles
- BACE1 elevation is involved in amyloid plaque development in the triple transgenic model of Alzheimer's disease: differential Aβ antibody labeling of early-onset axon terminal pathology.
Cai Y, Zhang XM, Macklin LN, Cai H, Luo XG, Oddo S, Laferla FM, Struble RG, Rose GM, Patrylo PR, Yan XX. Cai Y, et al. Neurotox Res. 2012 Feb;21(2):160-74. doi: 10.1007/s12640-011-9256-9. Epub 2011 Jul 2. Neurotox Res. 2012. PMID: 21725719 Free PMC article. - Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer's disease.
Sadleir KR, Kandalepas PC, Buggia-Prévot V, Nicholson DA, Thinakaran G, Vassar R. Sadleir KR, et al. Acta Neuropathol. 2016 Aug;132(2):235-256. doi: 10.1007/s00401-016-1558-9. Epub 2016 Mar 18. Acta Neuropathol. 2016. PMID: 26993139 Free PMC article. - β-Secretase-1 elevation in aged monkey and Alzheimer's disease human cerebral cortex occurs around the vasculature in partnership with multisystem axon terminal pathogenesis and β-amyloid accumulation.
Cai Y, Xiong K, Zhang XM, Cai H, Luo XG, Feng JC, Clough RW, Struble RG, Patrylo PR, Chu Y, Kordower JH, Yan XX. Cai Y, et al. Eur J Neurosci. 2010 Oct;32(7):1223-38. doi: 10.1111/j.1460-9568.2010.07376.x. Epub 2010 Aug 18. Eur J Neurosci. 2010. PMID: 20726888 Free PMC article. - BACE1: the beta-secretase enzyme in Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- BACE1 elevation engendered by GGA3 deletion increases β-amyloid pathology in association with APP elevation and decreased CHL1 processing in 5XFAD mice.
Kim W, Ma L, Lomoio S, Willen R, Lombardo S, Dong J, Haydon PG, Tesco G. Kim W, et al. Mol Neurodegener. 2018 Feb 2;13(1):6. doi: 10.1186/s13024-018-0239-7. Mol Neurodegener. 2018. PMID: 29391027 Free PMC article. - Temporal gene profiling of the 5XFAD transgenic mouse model highlights the importance of microglial activation in Alzheimer's disease.
Landel V, Baranger K, Virard I, Loriod B, Khrestchatisky M, Rivera S, Benech P, Féron F. Landel V, et al. Mol Neurodegener. 2014 Sep 11;9:33. doi: 10.1186/1750-1326-9-33. Mol Neurodegener. 2014. PMID: 25213090 Free PMC article. - BACE1 inhibition more effectively suppresses initiation than progression of β-amyloid pathology.
Peters F, Salihoglu H, Rodrigues E, Herzog E, Blume T, Filser S, Dorostkar M, Shimshek DR, Brose N, Neumann U, Herms J. Peters F, et al. Acta Neuropathol. 2018 May;135(5):695-710. doi: 10.1007/s00401-017-1804-9. Epub 2018 Jan 11. Acta Neuropathol. 2018. PMID: 29327084 Free PMC article. - Can brain impermeable BACE1 inhibitors serve as anti-CAA medicine?
Li JM, Huang LL, Liu F, Tang BS, Yan XX. Li JM, et al. BMC Neurol. 2017 Aug 25;17(1):163. doi: 10.1186/s12883-017-0942-y. BMC Neurol. 2017. PMID: 28841840 Free PMC article.
References
- Arendt T. Alzheimer's disease as a disorder of dynamic brain self-organization. Prog Brain Res. 2005;147:355–378. - PubMed
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. - PubMed
- Blanchard V, Moussaoui S, Czech C, Touchet N, Bonici B, Planche M, Canton T, Jedidi I, Gohin M, Wirths O, Bayer TA, Langui D, Duyckaerts C, Tremp G, Pradier L. Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Exp. Neurol. 2003;184:247–263. - PubMed
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 2001;4:233–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases