The Beclin 1 interactome - PubMed (original) (raw)
The Beclin 1 interactome
Congcong He et al. Curr Opin Cell Biol. 2010 Apr.
Abstract
The mammalian ortholog of yeast Atg6/Vps30, Beclin 1, is an essential autophagy protein that has been linked to diverse biological processes, including immunity, development, tumor suppression, lifespan extension, and protection against certain cardiac and neurodegenerative diseases. In recent years, major advances have been made in identifying components of functionally distinct Beclin 1/class III phosphatidylinositol 3-kinase complexes, in characterizing the molecular regulation of interactions between Beclin 1 and the autophagy inhibitors, Bcl-2/BcL-X(L), and in uncovering a role for viral antagonists of Beclin 1 in viral pathogenesis. The rapidly growing list of components of the 'Beclin 1 interactome' supports a model in which autophagy, and potentially other membrane trafficking functions of Beclin 1, are governed by differential interactions with different binding partners in different physiological or pathophysiological contexts.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Figure 1
Function of proteins that interact with Beclin 1/class III PI3K complexes in different steps of autophagy. Beclin 1, Vps34, Vps15, and possibly Ambra 1, compose a class III PI3K core complex that binds either Atg14 or UVRAG. Atg14 activates the core complex and biogenesis of autophagosomes; however, there are conflicting reports as to whether UVRAG/Bif-1 facilitates the core complex and upregulates autophagosome formation. A better studied function of UVRAG is promoting the maturation of autophagosomes and endocytic vesicles by recruiting class C Vps and the Rab7 GTPase and inducing vesicle fusion. As an important negative regulator of autophagy, Rubicon inhibits multiple steps in autophagy, including the formation of autophagosomes and the fusion between lysosomes and autophagosomes/endocytic vesicles. However, it is not yet known whether Rubicon inhibits autophagosome formation directly through an inhibitory interaction with the core complex or whether Beclin 1 is involved in the inhibitory effects of Rubicon on autophagosome/endosome maturation. Green arrows, stimulatory action; red bars, inhibitory action.
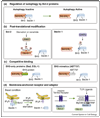
Figure 2
Mechanisms underlying the regulation of Bcl-2/Bcl-XL interactions with Beclin 1. (a) When Bcl-2/Bcl-XL are bound to Beclin 1, they inhibit autophagy. The disruption of Bcl-2/Bcl-XL–Beclin 1 interactions is essential for autophagy activation. Mechanisms by which this occurs include post-translational modifications of either Bcl-2/Bcl-XL or Beclin 1 (b); competitive disruption of Bcl-2/Bcl-XL binding to Beclin 1 by BH3-only proteins (e.g. Bad, EGL-1) or BH3 mimetics (e.g. ABT737) (c), or potentially, effects of membrane-anchored receptors or their adaptors on Bcl-2/Beclin 1 interactions (d). In (b), starvation induces JNK1-dependent phosphorylation of residues T69, S70, and S87 of Bcl-2, resulting in the disruption of Bcl-2 from Beclin 1. The death-associated kinase, DAPk, phosphorylates residue T119 in the BH3 domain of Beclin 1, leading to the disruption of Bcl-2 from Beclin 1. In (d), Beclin 1 is released from ER-localized inositol-1,4,5-trisphosphate receptor (IP3R) and Bcl-2 after antagonist binding or during starvation, leading to activation of autophagy. The activation of Toll-like receptors recruits the adaptor proteins MyD88 and TRIF, which interact with Beclin 1 and decrease the inhibitory interaction of Bcl-2 with Beclin 1. Green arrows, autophagy induction; red bars, autophagy inhibition.
Figure 3
The Beclin 1 viral protein interactome and their presumed sites of anti-autophagy action. HSV-1 ICP34.5 and γ-herpesvirus-encoded viral Bcl-2 molecules inhibit autophagosome formation through their interactions with Beclin 1, whereas HIV-1 Nef and influenza M2 block the maturation step of autophagy. The anti-autophagic maturation activity of HIV-1 Nef is thought to be mediated by interactions with Beclin 1 (see text), whereas it is not yet known whether the anti-autophagic maturation activity of influenza M2 is mediated by its interaction with Beclin 1.
Similar articles
- Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG.
Itakura E, Kishi C, Inoue K, Mizushima N. Itakura E, et al. Mol Biol Cell. 2008 Dec;19(12):5360-72. doi: 10.1091/mbc.e08-01-0080. Epub 2008 Oct 8. Mol Biol Cell. 2008. PMID: 18843052 Free PMC article. - Beclin 1 bridges autophagy, apoptosis and differentiation.
Wang J. Wang J. Autophagy. 2008 Oct;4(7):947-8. doi: 10.4161/auto.6787. Epub 2008 Oct 14. Autophagy. 2008. PMID: 18769161 - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond.
Funderburk SF, Wang QJ, Yue Z. Funderburk SF, et al. Trends Cell Biol. 2010 Jun;20(6):355-62. doi: 10.1016/j.tcb.2010.03.002. Epub 2010 Mar 29. Trends Cell Biol. 2010. PMID: 20356743 Free PMC article. Review. - The Beclin 1 network regulates autophagy and apoptosis.
Kang R, Zeh HJ, Lotze MT, Tang D. Kang R, et al. Cell Death Differ. 2011 Apr;18(4):571-80. doi: 10.1038/cdd.2010.191. Epub 2011 Feb 11. Cell Death Differ. 2011. PMID: 21311563 Free PMC article. Review.
Cited by
- Erythropoietin modulates autophagy signaling in the developing rat brain in an in vivo model of oxygen-toxicity.
Bendix I, Schulze C, Haefen Cv, Gellhaus A, Endesfelder S, Heumann R, Felderhoff-Mueser U, Sifringer M. Bendix I, et al. Int J Mol Sci. 2012 Oct 10;13(10):12939-51. doi: 10.3390/ijms131012939. Int J Mol Sci. 2012. PMID: 23202931 Free PMC article. - Regulation of actin nucleation and autophagosome formation.
Coutts AS, La Thangue NB. Coutts AS, et al. Cell Mol Life Sci. 2016 Sep;73(17):3249-63. doi: 10.1007/s00018-016-2224-z. Epub 2016 May 4. Cell Mol Life Sci. 2016. PMID: 27147468 Free PMC article. Review. - Mechanistic studies of cytotoxic activity of the mesoionic compound MIH 2.4Bl in MCF-7 breast cancer cells.
de Mascena Costa LA, Debnath D, Harmon AC, de Sousa Araújo S, da Silva Souza HD, de Athayde Filho PF, Wischral A, Adrião Gomes Filho M, Mathis JM. de Mascena Costa LA, et al. Oncol Lett. 2020 Sep;20(3):2291-2301. doi: 10.3892/ol.2020.11763. Epub 2020 Jun 19. Oncol Lett. 2020. PMID: 32782546 Free PMC article. - Cullin-RING ligases in regulation of autophagy.
Cui D, Xiong X, Zhao Y. Cui D, et al. Cell Div. 2016 Jun 10;11:8. doi: 10.1186/s13008-016-0022-5. eCollection 2016. Cell Div. 2016. PMID: 27293474 Free PMC article. Review. - Multiple functions of BCL-2 family proteins.
Hardwick JM, Soane L. Hardwick JM, et al. Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2):a008722. doi: 10.1101/cshperspect.a008722. Cold Spring Harb Perspect Biol. 2013. PMID: 23378584 Free PMC article. Review.
References
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. This work was the first to show that beclin 1 is an autophagy gene and tumor suppressor molecule.
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. This work was the first to show that mammalian beclin 1, like yeast ATG6/VPS30, controls autophagy as part of a class III PI3K complex.
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA084254-10S1/CA/NCI NIH HHS/United States
- R01 CA084254-10/CA/NCI NIH HHS/United States
- R01 CA084254/CA/NCI NIH HHS/United States
- R0I CA84254/CA/NCI NIH HHS/United States
- R01 CA109618-05/CA/NCI NIH HHS/United States
- R01 AI051367/AI/NIAID NIH HHS/United States
- R01 CA109618/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI051367-06/AI/NIAID NIH HHS/United States
- R0I CA109618/CA/NCI NIH HHS/United States
- R01 AI151267/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources