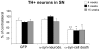Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson's disease - PubMed (original) (raw)
Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson's disease
Vanesa Sanchez-Guajardo et al. PLoS One. 2010.
Abstract
Post-mortem analysis of brains from Parkinson's disease (PD) patients strongly supports microglia activation and adaptive immunity as factors contributing to disease progression. Such responses may be triggered by alpha-synuclein (alpha-syn), which is known to be the main constituent of the aggregated proteins found in Lewy bodies in the brains of PD patients. To investigate this we used a recombinant viral vector to express human alpha-syn in rat midbrain at levels that induced neuronal pathology either in the absence or the presence of dopaminergic cell death, thereby mimicking early or late stages of the disease. Microglia activation was assessed by stereological quantification of Mac1+ cells, as well as the expression patterns of CD68 and MCH II. In our study, when alpha-syn induced neuronal pathology but not cell death, a fast transient increase in microglia cell numbers resulted in the long-term induction of MHC II+ microglia, denoting antigen-presenting ability. On the other hand, when alpha-syn induced both neuronal pathology and cell death, there was a delayed increase in microglia cell numbers, which correlated with long-lasting CD68 expression and a morphology reminiscent of peripheral macrophages. In addition T-lymphocyte infiltration, as judged by the presence of CD4+ and CD8+ cells, showed distinct kinetics depending on the degree of neurodegeneration, and was significantly higher when cell death occurred. We have thus for the first time shown that the microglial response differs depending on whether alpha-syn expression results on cell death or not, suggesting that microglia may play different roles during disease progression. Furthermore, our data suggest that the microglial response is modulated by early events related to alpha-syn expression in substantia nigra and persists at the long term.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
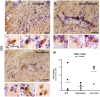
Figure 1. Tyrosine hydroxylase immunostaining.
TH immunohistochemical staining of striatal (A-I) and nigral sections (J-L) from animals of the GFP group (A, D, G and J), the α-syn-neurodegeneration group (B, E, H and K), or the α-syn-cell death group (C, F, I and L) at 4 weeks (A-C), 8 weeks (D-F) or 15 weeks (G-L) post- rAAV2/5 injection. GFP expressing animals showed dense dopaminergic fiber staining in striatum (A, D and G) and numerous TH+ neurons in SN (J) that did not change after 15 weeks. Animals in the α-syn-neurodegeneration group showed an apparent decrease in TH+ fiber staining in striatum at 8 weeks that further decreased at 15 weeks (E and H). After 8 weeks, numerous TH+ fibers appeared thicker and/or with small round formations, which became bigger after 15 weeks (arrows in E and H). However, the number of TH+ neurons in SN (K) remained similar to control levels (both contralateral side (not shown) and to GFP in J) after 15 weeks. In the cell death group α-syn expression lead to loss of TH fibers that was already apparent at 4 weeks; with time fibers became thicker and more apparent, although less numerous (C, F and I). TH+ fibers in these animals showed thickening and pathological round formations from early time points that increased with time (arrows in C, F and I). This was accompanied by a significant decrease of TH+ neurons of similar magnitude at 4, 8 (data not shown) and 15 weeks (L). Scale in I: 40 µm, applies to A-I. Scale in L**:** 100 µm, applies to J-L.
Figure 2. Dopaminergic cell survival in Substantia Nigra.
Graph illustrates the estimation of total dopaminergic neurons in SN (expressed as % of control, contralateral side values) using stereological tools in all groups and time points. A significant decrease of the number of TH+ cells was observed in the α-syn-cell death group at all time points. Two-way ANOVA [F (8,46) = 6.18, p<0.01] followed by individual contrast. One-way ANOVA: * <0.05 and ** <0.01 different from the GFP group at the same time point, effect of group at 4 weeks [F (2, 14) = 9.3, p<0.01], at 15 weeks [F (2, 16) = 9.3, p<0.01], at 15 weeks [F (2, 14) = 4.1, p<0.05] followed by a Dunnett's post-hoc analysis.
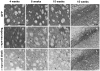
Figure 3. Transgene expression.
Transgene immunostaining at striatal (A–C, E–G and I–K) or nigral level (D, H and L) from animals of the GFP group (A–D), α-syn-neurodegenerative group (E–H), and α-syn-cell death group (I–L) at 4 weeks (A, E and I), 8 weeks (B, F and J), or 15 weeks (C, D, G, H, K and L) post- rAAV2/5 injection. GFP immunostaining was observed in the ipsilateral side of animals injected with GFP-rAAV2/5, as a dense fiber staining where no apparent pathology was observed (A, B and C). In the midbrain we observed neurons expressing GFP both in SNc (arrows) and SNr (arrowheads) that remained after 15 weeks (D). Animals in the α-syn-neurodegeneration group showed dense fiber immunostaining for human-α-syn at 4 weeks (B). Fiber staining decreased progressively with the concomitant appearance of pathological formations (F and J). At 8 weeks numerous round α-syn+ formations were observed (arrows in F). With time these formations became bigger (arrows in J) and thickening of α-syn+ fibers was observed (arrowheads in J). At the nigral level, numerous α-syn+ neurons in SNc persisted after 15 weeks (arrows in H). In animals of α-syn cell death group, fiber staining was lower than in the other α-syn group at 4 weeks (I vs. E). Progressively α-syn+ fibers became thicker and less abundant in striatum (arrowheads in I–K). In addition, α-syn+ pathological formations were already apparent at 4 weeks, they persisted and became bigger as time progressed (arrows in I–K). In the midbrain we observed α-syn+ neurons both in SNc (arrows) and SNr (arrowheads) that remained after 15 weeks (L). There was a lower number of α-syn+ neurons in SNc than in the α-syn-neurodegeneration group (L vs. H). Scale: 100 µm, in A applies to A–C, E–G and I–K; in D applies to D, H and L.
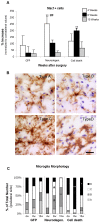
Figure 4. Microglia morphology and cell number.
(A) Animals expressing GFP, or α-syn at levels that caused either neurodegeneration or cell death were killed at 4 (white bars), 8 (black bars) and 15 (grey bars) weeks after surgery. SN resident microglia cell numbers and morphology were stereologically analyzed at each time point. Data represents average increase of Mac1+ microglia cell number in the ipsilateral SN as compared to the contralateral SN (n = 5–6) + S.E.M. Two-way ANOVA [F (8,45) = 11.2, p<0.0001] followed by Tukey HSD post-hoc analysis. * or # p<0.05 ** p<0.01 (*) compared to the GFP control group at same time point; (#) compared to the other α-syn group at same time point. (B) The four morphologies of microglia are represented: Type A, corresponding to resting microglia: cells with a round dense nucleus without a visible cytoplasm surrounding it, and with long thin processes. Type B: there is a visible small thin cytoplasm around a dense nucleus, processes remain thin, but are longer with many branches of less defined edges. Type C: the cell body becomes elongated and irregular, with an enlarged and less defined nucleus, processes become shorter of varying thickness and little branching. Type D: the cell body is big and dark, merging with thick short processes; the nucleus occupies most of the cell body and is not always distinguishable. Scale in type D: 20 µm, applies to all. (C) Stereological quantification of each morphology type is depicted as the average percentage distribution per group as a function of time. Two-way ANOVA, when significant followed by Tukey-Kramer post-hoc analysis. # p<0.05 compared to the other α-syn group at same time point; * p<0.05, ** p<0.01 compared to the other two groups at same time point.
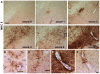
Figure 5. CD68 microglial expression.
The levels of CD68 expression were scored 0–3 (A): Score 0, non or 1–3 isolated positive cells; Score 1, few scattered cells; Score 2, throughout the SNc; Score 3, throughout the SN. Scale in Score 3: 80 µm, applies to all in A. (B) Higher magnification of representative CD68+ immunostaining. Notice how the intracellular vesicular localization of the protein (arrowheads) makes it difficult to determine how many positive dots belong to one microglia. Scale in B: 13 µm (top) and 20 µm (bottom).
Figure 6. MHC II microglial expression.
Photos show representative nigral sections immunostained for MHCII. The level of MHC II expression in microglia was scored 0–5 (A): Score 0, no positive cells; Score 1, few isolated cells; Score 2, scattered throughout the SNc or few clusters; Score 3, throughout the SNc; Score 4, throughout the SN; Score 5, saturation of the SN. (B) High magnification photo of the insert showed in A Score 4. The most abundant MHC II+ microglia morphology corresponded to microglia type B (arrow). (C) In the groups expressing α-syn, we also observed numerous small MHC II+ round cells at 4 (not shown) and 8 weeks. (D–E) MHC II+ cells in association with blood vessels. Occasionally we saw MHC II+ cells, which seemed to be inside of the vessel's lumen (arrowhead in D); in other cases MHC II+ cells seemed to penetrate and/or surround the vessel's wall (arrowheads in E). The images come from, C and D: neurodegeneration at 4 weeks (Score 5), E: neurodegeneration 8 weeks (Score 3). Scale in Score 0: 90 µm, applies to all in A. Scales in B: 10 µm, in C: 40 µm and in D&E: 20 µm.
Figure 7. T cell infiltration.
Photos show SN of animals immunostained with antibody against CD3 in order to address T cell infiltration (A–L). CD3+ cells were found either isolated (A), associated with blood vessels (B) or clustered together (C). Immunostaining appeared homogeneously distributed in the cell body (arrowheads in A and B) or punctuated (arrowheads in C). Scale: 40 µm, applies to A–C. The small panels show individual CD3+ cells from A (D–F), B (G–I) and C (J–K) at high magnification. (L) Panel shows high magnification of CD3+ cells with punctuated staining without counterstaining in order to appreciate better the staining pattern. Scale: 10 µm, applies to D–L. Photos were taken from sections of animals of the following groups, A: cell death 4 weeks, B: cell death 8 weeks, C+L: neurodegeneration 15 weeks. (D) Graph shows average (bar) and individual numbers of CD3+ cells found in one SN section at 8 weeks. These are plotted per animal in each group.
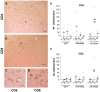
Figure 8. CD4 and CD8 T cell infiltration.
Photos show SN sections of an animal of the cell death group immunostained with antibody against CD4 (A and C) and CD8 (B and D). The small panels show insets in A (C) and in B (D) at higher magnification. Scale: 50 µm, applies to A–B, 10 µm applies to C–D. (E) Graph shows average (dash) and individual numbers of CD4+ cells found in one SN section per animal of each group plotted per time. Two-way ANOVA [F (8,42) = 4.1, p = 0.001 effect of group and time interaction] followed by Tukey HSD post-hoc analysis. ## or ç p<0.01 (##) compared to the other α-syn group at same time point; (††) different to the next time point of the same group. (F) Graph shows average (dash) and individual numbers of CD8+ cells found in one SN section per animal of each group plotted per time. Two-way ANOVA [F (8,41) = 4.3, p = 0.001 effect of group and time interaction] followed by Tukey HSD post-hoc analysis. (**) p<0.01 compared to all other groups at all time point.
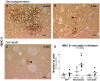
Figure 9. MHC II expression in the Striatum.
Photos show representative striatal sections immunostained for MHC II from animals in the α-syn neurodegeneration (A–B) or cell death group (C). In the neurodegeneration group we saw an up-regulation of the molecule throughout the striatum at 4 weeks (A). At 8 weeks, expression persisted but had diminished to several isolated cells or small clusters (B). In the cell death group, we only observed few isolated weakly stained positive cells at 8 weeks (C). Graph shows average (bar) and individual MHC II+ cells numbers found in one striatum section at 15 weeks. These are plotted per animal in each group (Contralateral vs. Ipsilateral) (D). One-way ANOVA when significant followed by Tukey-Kramer post-hoc analysis, (**) p<0.01 compared to ipsilateral site of the other two groups.
References
- Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Arch Neurol. 2005;62:353–357. - PubMed
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp Neurol. 2005;193:279–290. - PubMed
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. - PubMed
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous