CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC - PubMed (original) (raw)
CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC
Lara E Kallal et al. Eur J Immunol. 2010 Apr.
Abstract
Chemokines are important mediators of the immune response to pathogens, but can also promote chronic inflammatory states. Chemokine receptor 6 (CCR6) is found on immature DC and effector/memory T cells, and binds a single ligand, CCL20, with high affinity. Here, we investigated the role of CCL20 and CCR6 in a pulmonary viral infection caused by RSV, a ubiquitous virus that can cause severe pulmonary complications. Neutralization of CCL20 during RSV infection significantly reduced lung pathology and favored a Th1 effector response. CCR6-deficient animals recapitulated this phenotype, and additionally showed enhanced viral clearance when compared with WT mice. No differences were observed in migration of T cells to the lungs of CCR6(-/-) animals; however, a significant reduction was observed in numbers of conventional DC (cDC), but not plasmacytoid DC, in CCR6(-/-) mice. A pathogenic phenotype could be reconstituted in CCR6(-/-) mice by supplying cDC into the airway, indicating that mere number of cDC dictates the adverse response. Our data suggest that blockade of the CCL20/CCR6 pathway provides an environment whereby the attenuated recruitment of cDC alters the balance of innate immune cells and mediates the efficient antiviral response to RSV.
Figures
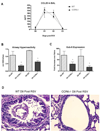
Figure 1. Less pathophysiology in CCR6−/− mice
A. Bronchoalveolar lavage (BAL) was performed on mice at 1, 2 and 3 days post infection with 5×104 PFU RSV/mouse and levels of CCL20 determined by ELISA. B. Mice were assessed for airway hyperreactivity after 6 and 9 days post-RSV infection by box plethysmography in anesthetized, ventilated animals. Peak airway resistance was recorded after tail vein administration of 200µg of methacholine. Data represent the mean ± SE from four mice per group. *P < 0.05 C. Left lobes were harvested from mice after 6 and 8 days of RSV infection and snap-frozen. RNA was extracted using Trizol and Gob-5 expression determined using quantitative RT-PCR. Data represent the mean ± SE from four mice/group/experiment, data are pooled from two experiments. *P < 0.05 D. Right lobes were harvested from mice after 8 days of RSV infection, fixed in formalin and sections stained with Periodic Acid Schiff to visualize mucus.
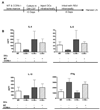
Figure 2. Altered T cell response in CCR6−/− mice
A., B. Whole lungs were harvested from mice after 8 days of RSV infection and single cell suspensions obtained using collagenase digestion. Samples were stained with FITC-CD4, PE-CD69 and PE-CY5-CD8 and analyzed on a Beckman Flow Cytometer. CD69+ CD4 (A) and CD8 (B) T cells were calculated as a percentage of total lymphocytes in each sample. Data represent the mean ± SE from four mice per group. *P < 0.05. C. Lymph nodes were collected at day 6 and 9 post-RSV infection and single cell suspensions obtained using a mesh filter. 1×106 cells/sample were incubated for 24 hours with 4×104 PFU RSV and supernatants collected. IL-4, IL-5, IL-13 and IFNγ levels were determined using Bioplex. Data is representative of both day 6 (shown) and day 9 and represents the mean ± SE from four mice per group. *P < 0.05.
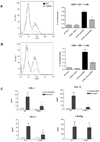
Figure 3. Differential dendritic cell recruitment in CCR6−/− mice
A. Peak RSV titer was determined after 3 days of RSV infection. Viral plaque assay was performed on whole lung supernatants and titer determined using an RSV-specific antibody. Data represent the mean ± SE from five mice per group. *P < 0.05. B., C. Dendritic cell subset recruitment in the lung was determined after 1 and 2 days post-RSV infection using combinations of the following antibodies: FITC-MHCII, APC-CD11c, PE-CY5-CD11B and PE-CY5-B220. Conventional DCs (B) and plasmacytoid DCs (C) are presented as total cells per lung. Data represent the mean ± SE from four mice per group. *P < 0.05. D. BAL was performed on mice infected with RSV for 1, 2 and 3 days and supernatants collected for analysis of chemokine production. CXCL10, CCL2 and CCL5 were measured using Bioplex. Data represent the mean ± SE from four mice per group. *P < 0.05.
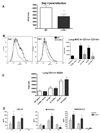
Figure 4. Bone-marrow derived DCs administered down the airways of CCR6−/− mice reconstitutes WT phenotype
A. Schematic representation of DC transfer experiment: WT and CCR6−/− DCs were differentiated from bone marrow and 5×105 intratracheally injected into WT and CCR6−/− mice. Mice were intranasally infected with RSV and lymph nodes removed 8 days later. B. Lymph nodes were harvested from WT and CCR6−/− mice 8 days post-RSV infection, as well as from CCR6−/− mice having received both WT and CCR6−/− DCs just prior to RSV infection. Restimulation with RSV was performed on lymph node cells and cytokines measured by Bioplex. Data represent the mean ± SE from five mice per group.
Figure 5. Enhanced capacity of CCR6−/− DCs to promote Th1 T cell responses
A. Bone marrow dendritic cells from WT and CCR6−/− mice were pulsed with 4×104 PFU RSV for 2 hrs and cultured with CD4+ T cells isolated by magnetic selection (MACS) from the lymph nodes of WT and CCR6−/− mice at 8 days post-RSV infection. After 48 hrs, supernatants were collected and IL-4 and IFNγ levels determined by Bioplex. Data represent the mean ± SE from three replicates per group. *P < 0.05 B. Bone marrow dendritic cells from WT and CCR6−/− mice were pulsed with 1µg/ml OVA peptide for 2 hrs, then cultured with CD4+ T cells isolated by magnetic selection from the spleens of DO11.10 mice. After 24 hrs, supernatants were collected and IL-4 and IFNγ levels determined by Bioplex. Data represent the mean ± SE from three replicates per group. *P < 0.05. C. 1×106 WT and CCR6−/− bone marrow dendritic cells were stimulated with 4×104 PFU RSV or 2.5µg/ml PolyI:C. Cells were harvested at 6 and 48 hours, resuspended in Trizol and quantitative RT-PCR performed to determine IFN-alpha and IFN-beta expression. Data represent the mean ± SE from three replicates per group. *P < 0.05.
Similar articles
- Respiratory Syncytial virus infection compromises asthma tolerance by recruiting interleukin-17A-producing cells via CCR6-CCL20 signaling.
Shi T, He Y, Sun W, Wu Y, Li L, Jie Z, Su X. Shi T, et al. Mol Immunol. 2017 Aug;88:45-57. doi: 10.1016/j.molimm.2017.05.017. Epub 2017 Jun 7. Mol Immunol. 2017. PMID: 28599122 - Respiratory syncytial virus infection modifies and accelerates pulmonary disease via DC activation and migration.
Jang S, Smit J, Kallal LE, Lukacs NW. Jang S, et al. J Leukoc Biol. 2013 Jul;94(1):5-15. doi: 10.1189/jlb.0412195. Epub 2013 Jan 4. J Leukoc Biol. 2013. PMID: 23293372 Free PMC article. - Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guérin promotes virus clearance and protects from infection.
Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Cautivo KM, et al. J Immunol. 2010 Dec 15;185(12):7633-45. doi: 10.4049/jimmunol.0903452. Epub 2010 Nov 17. J Immunol. 2010. PMID: 21084664 - CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis.
Williams IR. Williams IR. Ann N Y Acad Sci. 2006 Aug;1072:52-61. doi: 10.1196/annals.1326.036. Ann N Y Acad Sci. 2006. PMID: 17057190 Review. - CCR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis.
Lee AYS, Körner H. Lee AYS, et al. J Gen Virol. 2017 Mar;98(3):338-344. doi: 10.1099/jgv.0.000691. Epub 2017 Apr 1. J Gen Virol. 2017. PMID: 28005525 Review.
Cited by
- Site-specific dynamics of CD11b+ and CD103+ dendritic cell accumulations following ozone exposure.
Brand JD, Ballinger CA, Tuggle KL, Fanucchi MV, Schwiebert LM, Postlethwait EM. Brand JD, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Dec 15;303(12):L1079-86. doi: 10.1152/ajplung.00185.2012. Epub 2012 Oct 19. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 23087018 Free PMC article. - TLR1-induced chemokine production is critical for mucosal immunity against Yersinia enterocolitica.
Sugiura Y, Kamdar K, Khakpour S, Young G, Karpus WJ, DePaolo RW. Sugiura Y, et al. Mucosal Immunol. 2013 Nov;6(6):1101-9. doi: 10.1038/mi.2013.5. Epub 2013 Feb 27. Mucosal Immunol. 2013. PMID: 23443468 Free PMC article. - Functional Impairment of Mononuclear Phagocyte System by the Human Respiratory Syncytial Virus.
Bohmwald K, Espinoza JA, Pulgar RA, Jara EL, Kalergis AM. Bohmwald K, et al. Front Immunol. 2017 Nov 27;8:1643. doi: 10.3389/fimmu.2017.01643. eCollection 2017. Front Immunol. 2017. PMID: 29230219 Free PMC article. Review. - Central role of dendritic cells in shaping the adaptive immune response during respiratory syncytial virus infection.
McDermott DS, Weiss KA, Knudson CJ, Varga SM. McDermott DS, et al. Future Virol. 2011 Aug;6(8):963-973. doi: 10.2217/fvl.11.62. Future Virol. 2011. PMID: 21887154 Free PMC article. - Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection.
Reed M, Morris SH, Jang S, Mukherjee S, Yue Z, Lukacs NW. Reed M, et al. J Immunol. 2013 Sep 1;191(5):2526-37. doi: 10.4049/jimmunol.1300477. Epub 2013 Jul 26. J Immunol. 2013. PMID: 23894198 Free PMC article.
References
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. - PubMed
- John AE, Berlin AA, Lukacs NW. Respiratory syncytial virus-induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur J Immunol. 2003;33:1677–1685. - PubMed
- Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. - PubMed
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI036302/AI/NIAID NIH HHS/United States
- K22 AI077712/AI/NIAID NIH HHS/United States
- R29 AI036302/AI/NIAID NIH HHS/United States
- R01 AI073876/AI/NIAID NIH HHS/United States
- R01 AI036302-14/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases