Running reduces stress and enhances cell genesis in aged mice - PubMed (original) (raw)
Comparative Study
Running reduces stress and enhances cell genesis in aged mice
Timal S Kannangara et al. Neurobiol Aging. 2011 Dec.
Abstract
Cell proliferation and neurogenesis are diminished in the aging mouse dentate gyrus. However, it is not known whether isolated or social living affects cell genesis and stress levels in old animals. To address this question, aged (17-18 months old) female C57Bl/6 mice were single or group housed, under sedentary or running conditions. We demonstrate that both individual and socially housed aged C57Bl/6 mice have comparable basal cell proliferation levels and demonstrate increased running-induced cell genesis. To assess stress levels in young and aged mice, corticosterone (CORT) was measured at the onset of the active/dark cycle and 4h later. In young mice, no differences in CORT levels were observed as a result of physical activity or housing conditions. However, a significant increase in stress in socially housed, aged sedentary animals was observed at the onset of the dark cycle; CORT returned to basal levels 4h later. Together, these results indicate that voluntary exercise reduces stress in group housed aged animals and enhances hippocampal cell proliferation.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest
The authors have no current or potential conflicts of interest to report.
Figures
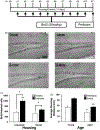
Fig. 1.
Voluntary exercise promotes cellular proliferation, irrespective of housing condition, in the aged mouse dentate gyrus. (A) Schematic diagram of the experimental paradigm and BrdU injection protocol. Exercising mice were allowed access to running wheels for 11 days. BrdU (50 mg/kg) was administered daily and mice were perfused on day 11. (B) Representative pictures of BrdU-positive cells in the dentate gyrus of individual sedentary controls (I-CON), individual runners (I-RUN), social sedentary controls (S-CON), and social runners (S-RUN). Scale bar: 50 μm. (C) Individual and social exercising groups (black bars) demonstrate increases in the amount of BrdU-positive cells in comparison to their sedentary controls (white bars). (*) Denotes significance (p < 0.05) in comparison to sedentary controls; (**) denotes significance (p < 0.05) in comparison to all other experimental groups. (D) Average running distance per cage during the 11-day exercise period for young and aged, individually and socially housed running animals. (*) and (**) denote significance (p < 0.05) in comparison to young individual runners and young social runners, respectively. Error bars represent S.E.M.
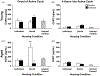
Fig. 2.
Corticosterone levels of young and aged mice. (A, B) In young mice no change in CORT was observed as a result of housing or exercise conditions. (C) Aged, socially housed, sedentary mice, have significantly higher stress levels than all other aged mice at active cycle onset. In addition, aged group housed runners have lower CORT levels than their young counterparts. (D) Four hours into active cycle, CORT levels of aged animals show no change as a result of exercise or housing condition. CORT levels in young Internal Controls that did not receive BrdU injections, are depicted with both young (A, B) and aged (C, D) groups. Error bars represent S.E.M. (**) Denotes significance in comparison to all other aged groups at the onset of the active cycle; (*) denotes a significant difference between young and aged grouped runners (p < 0.05).
Similar articles
- Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice.
Kannangara TS, Webber A, Gil-Mohapel J, Christie BR. Kannangara TS, et al. Hippocampus. 2009 Oct;19(10):889-97. doi: 10.1002/hipo.20514. Hippocampus. 2009. PMID: 18958850 - Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis.
Grégoire CA, Bonenfant D, Le Nguyen A, Aumont A, Fernandes KJ. Grégoire CA, et al. PLoS One. 2014 Jan 23;9(1):e86237. doi: 10.1371/journal.pone.0086237. eCollection 2014. PLoS One. 2014. PMID: 24465980 Free PMC article. - Cessation of voluntary wheel running increases anxiety-like behavior and impairs adult hippocampal neurogenesis in mice.
Nishijima T, Llorens-Martín M, Tejeda GS, Inoue K, Yamamura Y, Soya H, Trejo JL, Torres-Alemán I. Nishijima T, et al. Behav Brain Res. 2013 May 15;245:34-41. doi: 10.1016/j.bbr.2013.02.009. Epub 2013 Feb 18. Behav Brain Res. 2013. PMID: 23428744 - [Voluntary wheel running enhances cell proliferation and expression levels of BDNF, IGF1 and WNT4 in dentate gyrus of adult mice].
Yu JL, Ma L, Ma L, Tao YZ. Yu JL, et al. Sheng Li Xue Bao. 2014 Oct 25;66(5):559-68. Sheng Li Xue Bao. 2014. PMID: 25332001 Chinese. - Voluntary wheel running in mice increases the rate of neurogenesis without affecting anxiety-related behaviour in single tests.
Garrett L, Lie DC, Hrabé de Angelis M, Wurst W, Hölter SM. Garrett L, et al. BMC Neurosci. 2012 Jun 8;13:61. doi: 10.1186/1471-2202-13-61. BMC Neurosci. 2012. PMID: 22682077 Free PMC article.
Cited by
- Ageing, Cognitive Decline, and Effects of Physical Exercise: Complexities, and Considerations from Animal Models.
Caruso MG, Nicolas S, Lucassen PJ, Mul JD, O'Leary OF, Nolan YM. Caruso MG, et al. Brain Plast. 2024 May 14;9(1-2):43-73. doi: 10.3233/BPL-230157. eCollection 2024. Brain Plast. 2024. PMID: 38993577 Free PMC article. Review. - Aerobic exercise reverses aging-induced depth-dependent decline in cerebral microcirculation.
Shin P, Pian Q, Ishikawa H, Hamanaka G, Mandeville ET, Guo S, Fu B, Alfadhel M, Allu SR, Şencan-Eğilmez I, Li B, Ran C, Vinogradov SA, Ayata C, Lo E, Arai K, Devor A, Sakadžić S. Shin P, et al. Elife. 2023 Jul 4;12:e86329. doi: 10.7554/eLife.86329. Elife. 2023. PMID: 37402178 Free PMC article. - Cell-type-specific aging clocks to quantify aging and rejuvenation in neurogenic regions of the brain.
Buckley MT, Sun ED, George BM, Liu L, Schaum N, Xu L, Reyes JM, Goodell MA, Weissman IL, Wyss-Coray T, Rando TA, Brunet A. Buckley MT, et al. Nat Aging. 2023 Jan;3(1):121-137. doi: 10.1038/s43587-022-00335-4. Epub 2022 Dec 19. Nat Aging. 2023. PMID: 37118510 Free PMC article. - Pathophysiological basis and promise of experimental therapies for Gulf War Illness, a chronic neuropsychiatric syndrome in veterans.
Kodali M, Jankay T, Shetty AK, Reddy DS. Kodali M, et al. Psychopharmacology (Berl). 2023 Apr;240(4):673-697. doi: 10.1007/s00213-023-06319-5. Epub 2023 Feb 15. Psychopharmacology (Berl). 2023. PMID: 36790443 Review. - Exercise Duration Differentially Effects Age-related Neuroinflammation and Hippocampal Neurogenesis.
Connolly MG, Bruce SR, Kohman RA. Connolly MG, et al. Neuroscience. 2022 May 10;490:275-286. doi: 10.1016/j.neuroscience.2022.03.022. Epub 2022 Mar 21. Neuroscience. 2022. PMID: 35331843 Free PMC article.
References
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER, 1999. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. U.S.A 96, 5280–5285. - PMC - PubMed
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP, 2010. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging 31, 151–161. - PubMed
- Blackmore DG, Golmohammadi MG, Large B, Waters MJ, Rietze RL, 2009. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cells 27, 2044–2052. - PubMed
- Cameron HA, McKay RD, 1999. Restoring production of hippocampal neurons in old age. Nat. Neurosci 2, 894–897. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical