Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration - PubMed (original) (raw)
Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration
Jonathan M Bisaillon et al. Am J Physiol Cell Physiol. 2010 May.
Abstract
We recently demonstrated that thapsigargin-induced passive store depletion activates Ca(2+) entry in vascular smooth muscle cells (VSMC) through stromal interaction molecule 1 (STIM1)/Orai1, independently of transient receptor potential canonical (TRPC) channels. However, under physiological stimulations, despite the ubiquitous depletion of inositol 1,4,5-trisphosphate-sensitive stores, many VSMC PLC-coupled agonists (e.g., vasopressin and endothelin) activate various store-independent Ca(2+) entry channels. Platelet-derived growth factor (PDGF) is an important VSMC promigratory agonist with an established role in vascular disease. Nevertheless, the molecular identity of the Ca(2+) channels activated by PDGF in VSMC remains unknown. Here we show that inhibitors of store-operated Ca(2+) entry (Gd(3+) and 2-aminoethoxydiphenyl borate at concentrations as low as 5 microM) prevent PDGF-mediated Ca(2+) entry in cultured rat aortic VSMC. Protein knockdown of STIM1, Orai1, and PDGF receptor-beta (PDGFRbeta) impaired PDGF-mediated Ca(2+) influx, whereas Orai2, Orai3, TRPC1, TRPC4, and TRPC6 knockdown had no effect. Scratch wound assay showed that knockdown of STIM1, Orai1, or PDGFRbeta inhibited PDGF-mediated VSMC migration, but knockdown of STIM2, Orai2, and Orai3 was without effect. STIM1, Orai1, and PDGFRbeta mRNA levels were upregulated in vivo in VSMC from balloon-injured rat carotid arteries compared with noninjured control vessels. Protein levels of STIM1 and Orai1 were also upregulated in medial and neointimal VSMC from injured carotid arteries compared with noninjured vessels, as assessed by immunofluorescence microscopy. These results establish that STIM1 and Orai1 are important components for PDGF-mediated Ca(2+) entry and migration in VSMC and are upregulated in vivo during vascular injury and provide insights linking PDGF to STIM1/Orai1 during neointima formation.
Figures
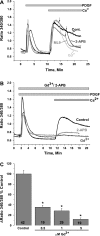
Fig. 1.
A: in the absence of extracellular Ca2+, platelet-derived growth factor (PDGF, 50 ng/ml) activates Ca2+ release from internal stores in primary cultured vascular smooth muscle cells (VSMC). Upon Ca2+ restoration to the extracellular milieu (2 mM), PDGF activates a Ca2+ entry pathway (cont, average of results from 18 cells) that is sensitive to 5 μM Gd3+ (n = 8), 30 μM 2-aminoethoxydiphenyl borate (2-APB, n = 29), and 50 μM ML-9 (n = 18). Arrows indicate addition of drugs. Values are expressed as ratio of fluorescence at 340 nm to fluorescence at 380 nm. B: preincubation with 5 μM Gd3+ (n = 10) or 5 μM 2-APB (n = 18) essentially abrogated PDGF-activated Ca2+ entry; n = 15 for control. C: dose-response inhibition of PDGF-mediated Ca2+ entry by Gd3+. Note substantial inhibition of Ca2+ entry at 0.5 μM Gd3+. Numbers within bars represent total number of cells. All Ca2+ imaging traces show average data from several cells analyzed simultaneously. Data are representative of 3–4 independent experiments. *P < 0.05.
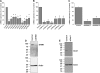
Fig. 2.
A–C: quantitative RT-PCR assessment of mRNA levels of stromal interaction molecule 1 (STIM) and Orai, transient receptor potential canonical (TRPC) isoforms, and PDGF receptor-β (PDGFRβ, C) after knockdown by transfection of 2 specific silent RNA (siRNA) sequences used independently or a scrambled siRNA sequence used as a control. After 72 h, mRNA was isolated from cells and reverse transcribed, and quantitative PCR was performed using specific primers. Sequences for siRNA and primers for STIM, TRPC, and Orai are provided elsewhere (34); sequences for primers and siRNA against PDGFRβ are provided in
methods
. Data are representative of 3 independent transfections performed in triplicates. *P < 0.05. D and E: Western blots showing STIM1 and Orai1 protein knockdown after siRNA transfection as previously reported (34). Data are representative of 4 independent experiments.
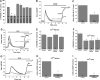
Fig. 3.
Representative traces showing average of PDGF-activated Ca2+ entry from several cells on single coverslips from scrambled control- or targeted siRNA-transfected VSMC on the same day. Results obtained with targeted siRNA against STIM1 and Orai1 (A), Orai2 (B), Orai3 (C), TRPC1 (D), TRPC4 (E), and TRPC6 (F) are shown. PDGF was used at 50 ng/ml for siOrai2 and at 25 ng/ml for all other recordings. Data are representative of 3–6 independent experiments per targeted siRNA. PDGF was first added in the absence of extracellular Ca2+; arrows show exact times of Ca2+ restoration to the extracellular milieu for each trace.
Fig. 4.
A: PDGF-activated Ca2+ entry after VSMC transfection with scrambled or targeted siRNA (against STIM1, Orai1, Orai2, Orai3, TRPC1, TRPC4, and TRPC6) was measured using fura 2, and extent of Ca2+ entry was analyzed in several cells originating from several independent experiments and is represented as the difference between the fura 2 signal before and after Ca2+ (2 mM) restoration. Results are averages of cells from 3–6 independent experiments per condition. Numbers within bars represent total number of cells for each condition. B: representative traces depicting effect of STIM1 and Orai1 simultaneous silencing on PDGF-mediated Ca2+ entry (n = 15). Condition with control scrambled siRNA-transfected cells (n = 22) is also shown. C: extent of Ca2+ entry in many scrambled control- and siOrai1 + siSTIM1-transfected cells. Results are averages 3 independent experiments; numbers within bars represent total number of cells. D: representative traces depicting effect of Orai1 (n = 15), siOrai1 + siOrai2 (n = 18), and siOrai1 + siOrai3 (n = 14) knockdown on PDGF-mediated Ca2+ entry compared with control scrambled siRNA-transfected cells (n = 20). E and F: Ca2+ release and entry in scrambled control-, siOrai1-, siOrai1 + siOrai2-, and siOrai1 + siOrai3-transfected cells. Results are averages from 3–4 independent experiments; numbers within bars represent total number of cells. G: representative traces depicting effect of PDGFRβ knockdown on PDGF-mediated Ca2+ release and entry (n = 16); control trace represents an average of 25 scrambled siRNA-transfected cells. H and I: Ca2+ release and entry in scrambled control- and PDGFRβ-transfected cells. Results are averages from 4 independent experiments; numbers within bars represent total number of cells. In all experiments, PDGF was used at a concentration of 50 ng/ml. *P < 0.05 vs. control for A, C, F, H, and I.
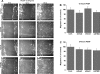
Fig. 5.
A: bright-field views of scratch wound migration assay in serum-free medium containing 100 ng/ml PDGF at 0–24 h after the wound in primary cultured VSMC transfected with scrambled control siRNA or siRNA against STIM1 or Orai1. B and C: data from 3 independent experiments (with 4 wells per condition) at 12 and 24 h after the wound. *P < 0.05.
Fig. 6.
A: bright-field views of scratch wound migration assay in serum-free medium containing 100 ng/ml PDGF at 0–24 h after the wound in primary cultured VSMC transfected with scrambled control siRNA or siRNA against STIM2, Orai2, or Orai3. B and C: data from 3 independent experiments (with 4 wells per condition) at 12 and 24 h after the wound.
Fig. 7.
A: bright-field views of scratch wound migration assay in serum-free medium containing 100 ng/ml PDGF at 0–24 h after the wound in primary cultured VSMC transfected with scrambled control siRNA or siRNA against PDGFRβ. B and C: data from 2 independent experiments (with 4 wells per condition) at 12 and 24 h after the wound. *P < 0.05. Data were generated using PDGFRβ siRNA1; similar results were obtained with PDGFRβ siRNA2 (not shown).
Fig. 8.
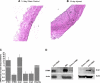
A and B: hematoxylin-eosin-stained sections of carotid arteries from a time-matched (14 days) noninjured sham control rat and a balloon-injured rat (14 days after injury). M, media; NI, neointima; L, lumen. C: quantitative RT-PCR assessment of mRNA levels of STIM and Orai isoforms and PDGFRβ. mRNAs were isolated from VSMC from sham control and injured rat carotid arteries. Values are expressed as fold increase in mRNA in injured carotid arteries compared with sham control. Data were obtained from 3 control and 3 injured rats that were analyzed in 3–7 independent runs. D: Western blots showing higher levels of STIM1 and Orai1 protein expression in synthetic (cultured) VSMC than in quiescent freshly isolated VSMC, as demonstrated previously (34). Rat basophilic leukemia (RBL) cells were used in parallel as a positive control.
Fig. 9.
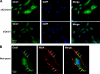
A: VSMC were transfected with nontargeting siRNA (siControl) or siRNA targeting Orai1 (siOrai1), fixed, and processed for immunofluorescence under permeabilized conditions using anti-Orai1; 4′,6′-diamidino-2-phenylindole (DAPI) was used as a nuclear marker. Images are representative of 3 independent experiments. A reduction in Orai1 expression is clearly detected in siOrai1 images. B: VSMC were fixed and processed for immunofluorescence under nonpermeabilized conditions (without Triton X-100) using anti-Orai1 (Orai1), wheat germ agglutinin (WGA)-Alexa Fluor 594 was used as a plasma membrane marker and DAPI as a nuclear marker, and images were collected using confocal microscopy. Arrows indicate colocalization between Orai1 and WGA at the plasma membrane (Merge, yellow staining). Images were modified in Adobe Photoshop to a higher level of contrast for better visualization.
Fig. 10.
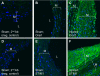
Immunofluorescence staining of carotid artery sections from rats subjected to balloon injury (C and F; 14 days after injury) and control noninjured vessels from sham-operated animals (A, B, D, and E) using anti-Orai1 (B and C) and anti-STIM1 (E and F) antibodies followed by a secondary antibody coupled to FITC. A and D: secondary antibody-alone control. Brackets indicate neointima (NI) in injured vessels. Data are representative of results obtained independently from sections originating from 3 control and 3 injured rats. M, media; L, lumen.
Fig. 11.
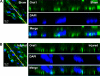
Carotid artery sections from rats subjected to balloon injury (B; 14 days after injury) and control noninjured vessels from sham-operated animals (A) were processed for immunofluorescence staining using anti-Orai1; DAPI was used as a nuclear marker. Vertical sections were collected at 0.5-μm intervals using confocal microscopy at high magnification. A representative image is shown at right. ImageJ Volume Viewer plug-in was used to generate a vertical cross section (yellow line) of the tissue slice. Images were modified in Adobe Photoshop to a higher level of contrast for better visualization. Orai1 shows a peripheral subcellular localization, which is consistent with a plasma membrane association.
Similar articles
- Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration.
Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Potier M, et al. FASEB J. 2009 Aug;23(8):2425-37. doi: 10.1096/fj.09-131128. Epub 2009 Apr 13. FASEB J. 2009. PMID: 19364762 Free PMC article. - Airway smooth muscle STIM1 and Orai1 are upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC currents, proliferation, and migration.
Spinelli AM, González-Cobos JC, Zhang X, Motiani RK, Rowan S, Zhang W, Garrett J, Vincent PA, Matrougui K, Singer HA, Trebak M. Spinelli AM, et al. Pflugers Arch. 2012 Nov;464(5):481-92. doi: 10.1007/s00424-012-1160-5. Epub 2012 Sep 27. Pflugers Arch. 2012. PMID: 23014880 Free PMC article. - Store-independent Orai1/3 channels activated by intracrine leukotriene C4: role in neointimal hyperplasia.
González-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M. González-Cobos JC, et al. Circ Res. 2013 Mar 29;112(7):1013-25. doi: 10.1161/CIRCRESAHA.111.300220. Epub 2013 Jan 24. Circ Res. 2013. PMID: 23349245 Free PMC article. - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
Cited by
- Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling.
Derouiche S, Warnier M, Mariot P, Gosset P, Mauroy B, Bonnal JL, Slomianny C, Delcourt P, Prevarskaya N, Roudbaraki M. Derouiche S, et al. Springerplus. 2013 Dec;2(1):54. doi: 10.1186/2193-1801-2-54. Epub 2013 Feb 15. Springerplus. 2013. PMID: 23450760 Free PMC article. - The mitochondrial regulation of smooth muscle cell proliferation in type 2 diabetes.
Koval OM, Nguyen EK, Mittauer DJ, Ait-Aissa K, Chinchankar W, Qian L, Madesh M, Dai DF, Grumbach IM. Koval OM, et al. bioRxiv [Preprint]. 2023 Feb 16:2023.02.15.528765. doi: 10.1101/2023.02.15.528765. bioRxiv. 2023. PMID: 36824758 Free PMC article. Updated. Preprint. - RAC1-Dependent ORAI1 Translocation to the Leading Edge Supports Lamellipodia Formation and Directional Persistence.
Lopez-Guerrero AM, Espinosa-Bermejo N, Sanchez-Lopez I, Macartney T, Pascual-Caro C, Orantos-Aguilera Y, Rodriguez-Ruiz L, Perez-Oliva AB, Mulero V, Pozo-Guisado E, Martin-Romero FJ. Lopez-Guerrero AM, et al. Sci Rep. 2020 Apr 20;10(1):6580. doi: 10.1038/s41598-020-63353-5. Sci Rep. 2020. PMID: 32313105 Free PMC article. - Altered Calcium Influx Pathways in Cancer-Associated Fibroblasts.
Sadras F, Stewart TA, Robitaille M, Peters AA, Croft PK, Soon PS, Saunus JM, Lakhani SR, Roberts-Thomson SJ, Monteith GR. Sadras F, et al. Biomedicines. 2021 Jun 16;9(6):680. doi: 10.3390/biomedicines9060680. Biomedicines. 2021. PMID: 34208665 Free PMC article. - Orai1 calcium channels in the vasculature.
Beech DJ. Beech DJ. Pflugers Arch. 2012 Apr;463(5):635-47. doi: 10.1007/s00424-012-1090-2. Epub 2012 Mar 9. Pflugers Arch. 2012. PMID: 22402985 Free PMC article. Review.
References
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium 33: 345–356, 2003 - PubMed
- Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 42: 213–223, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K22 ES-014729S2/ES/NIEHS NIH HHS/United States
- R01 HL049426/HL/NHLBI NIH HHS/United States
- K22 ES014729/ES/NIEHS NIH HHS/United States
- K22 ES-014729/ES/NIEHS NIH HHS/United States
- R01 HL097111/HL/NHLBI NIH HHS/United States
- K22 ES-014729S1/ES/NIEHS NIH HHS/United States
- R01 HL092510/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous