A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming - PubMed (original) (raw)
A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming
Ethan R Buch et al. J Neurosci. 2010.
Abstract
Ventral premotor cortex (PMv) is widely accepted to exert an important influence over primary motor cortex (M1) when hand movements are made. Although study of these interactions has typically focused on their excitatory nature, given its strong connections with both ventral and opercular frontal regions, one feature of the influence of PMv over M1 may be inhibitory. Paired-pulse transcranial magnetic stimulation (ppTMS) was used to examine functional interactions between human PMv and M1 during the selection and reprogramming of a naturalistic goal-directed action. One of two cylinders was illuminated on each trial. It was then grasped and picked up. On some trials, however, subjects had to reprogram the action as the illuminated cylinder was switched off and the other illuminated simultaneously with reach initiation. At a neurophysiological level, the PMv paired-pulse effect (PPE) on M1 corticospinal activity was facilitatory after the initial target presentation and during movement initiation. When reprogramming was required, however, the PPE became strongly inhibitory. This context-dependent change from facilitation to inhibition occurred within 75 ms of the change of target. Behaviorally, PMv-M1 ppTMS disrupted reprogramming. Diffusion-weighted magnetic resonance image scans were taken of each subject. Intersubject differences in the facilitation-inhibition contrast of PMv-M1 interactions were correlated with fractional anisotropy of white-matter in ventral prefrontal, premotor, and intraparietal brain areas. These results suggest that a network of brain areas centered on PMv inhibits M1 corticospinal activity associated with undesired movements when action plans change.
Figures
Figure 1.
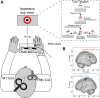
A, Behavioral task. spTMS or ppTMS was applied during most trials while subjects responded with their right hand (large cylinder stay trial shown in example). Either the large or small cylinder illuminated to initiate each trial. On stay trials the same cylinder remained illuminated throughout the trial. On switch trials, the illuminated cylinder switched from small-to-large or large-to-small when the subject's hand released the touch-bar. B, MNI coordinates for TMS targets. Circular symbols indicate individual subjects' stimulation locations in MNI152 space. Ellipsoids represent 95% confidence limits of the mean group stimulation location for each area [left M1: _X_= −36.5 ± 7.0 (mean ± SD), _Y_= −13.1 ± 6.9, Z = 64.2 ± 6.3; right PMv: X = 55.4 ± 4.0, Y = 14.7 ± 4.7, Z = 29.5 ± 4.6].
Figure 2.
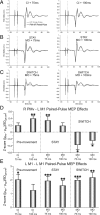
PMv-M1 ppTMS facilitated M1 corticospinal activity during the premovement period and in the perimovement stay condition. However, the PPE switched to perimovement inhibition when the action had to be inhibited and reprogrammed on switch trials. A–C, Example FDI MEPs recorded after M1 spTMS and PMv-M1 ppTMS during (A) premovement, and perimovement (B) stay and (C) switch conditions. D, Group mean PPEs for each condition in the FDI muscle. E, A nonspecific facilitatory PPE was observed across all conditions when M1–M1 ppTMS was applied. Error bars represent SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 3.
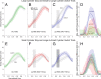
A–D, PMv-M1 ppTMS led to slower reprogramming on small-to-large cylinder switch trials (A), relative to the spTMS (B) or no-TMS conditions (C). This delay is quantified by the |stay − switch| GAD (D). E–H, Similar, albeit nonsignificant, trends were observed for reprogramming on large-to-small cylinder switch trials. Solid lines represent the group mean grasp aperture throughout the movement, with dashed lines representing the 95% CI of the mean. The hatched box represents the 95% CI of the mean occurrence of TMS stimulation following time normalization.
Figure 4.
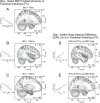
A–E, White-matter FA relationships with physiological (A–C) and behavioral (D–E) effects produced by PMv-M1 ppTMS. Clusters highlighting regions that showed differential PPEs relative to the behavioral context (stay vs switch) emerged in (A) PFv at MO +75 ms, and (B) PMv and (C) IPS at MO +100 ms. Similar (D) PMv and (E) IPS clusters emerge when FA is regressed against GAD measured at 50% of MT (∼300–400 ms after the MO +100 ms time-point). Correlation clusters are indicated by red-yellow colored voxels, with green cross-hairs through the center-of-mass. Inset scatter-plots depict the FA and regressor relationships for each cluster.
Similar articles
- The differential modulation of the ventral premotor-motor interaction during movement initiation is deficient in patients with focal hand dystonia.
Houdayer E, Beck S, Karabanov A, Poston B, Hallett M. Houdayer E, et al. Eur J Neurosci. 2012 Feb;35(3):478-85. doi: 10.1111/j.1460-9568.2011.07960.x. Eur J Neurosci. 2012. PMID: 22288483 Free PMC article. - Cortical and subcortical interactions during action reprogramming and their related white matter pathways.
Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Neubert FX, et al. Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13240-5. doi: 10.1073/pnas.1000674107. Epub 2010 Jul 9. Proc Natl Acad Sci U S A. 2010. PMID: 20622155 Free PMC article. Clinical Trial. - Inhibition of the anterior intraparietal area and the dorsal premotor cortex interfere with arbitrary visuo-motor mapping.
Taubert M, Dafotakis M, Sparing R, Eickhoff S, Leuchte S, Fink GR, Nowak DA. Taubert M, et al. Clin Neurophysiol. 2010 Mar;121(3):408-13. doi: 10.1016/j.clinph.2009.11.011. Epub 2009 Dec 9. Clin Neurophysiol. 2010. PMID: 20004613 - Motor areas in the frontal lobe of the primate.
Dum RP, Strick PL. Dum RP, et al. Physiol Behav. 2002 Dec;77(4-5):677-82. doi: 10.1016/s0031-9384(02)00929-0. Physiol Behav. 2002. PMID: 12527018 Review. - The primary motor and premotor areas of the human cerebral cortex.
Chouinard PA, Paus T. Chouinard PA, et al. Neuroscientist. 2006 Apr;12(2):143-52. doi: 10.1177/1073858405284255. Neuroscientist. 2006. PMID: 16514011 Review.
Cited by
- A Short Route for Reach Planning between Human V6A and the Motor Cortex.
Breveglieri R, Borgomaneri S, Diomedi S, Tessari A, Galletti C, Fattori P. Breveglieri R, et al. J Neurosci. 2023 Mar 22;43(12):2116-2125. doi: 10.1523/JNEUROSCI.1609-22.2022. Epub 2023 Feb 14. J Neurosci. 2023. PMID: 36788027 Free PMC article. - Neuroplasticity and network connectivity of the motor cortex following stroke: A transcranial direct current stimulation study.
Hordacre B, Moezzi B, Ridding MC. Hordacre B, et al. Hum Brain Mapp. 2018 Aug;39(8):3326-3339. doi: 10.1002/hbm.24079. Epub 2018 Apr 14. Hum Brain Mapp. 2018. PMID: 29655257 Free PMC article. Clinical Trial. - GABA Concentration in the Left Ventral Premotor Cortex Associates With Sensory Hyper-Responsiveness in Autism Spectrum Disorders Without Intellectual Disability.
Umesawa Y, Atsumi T, Chakrabarty M, Fukatsu R, Ide M. Umesawa Y, et al. Front Neurosci. 2020 May 19;14:482. doi: 10.3389/fnins.2020.00482. eCollection 2020. Front Neurosci. 2020. PMID: 32508576 Free PMC article. - Frontal and parietal cortex contributions to action modification.
Mutha PK, Stapp LH, Sainburg RL, Haaland KY. Mutha PK, et al. Cortex. 2014 Aug;57:38-50. doi: 10.1016/j.cortex.2014.03.005. Epub 2014 Mar 28. Cortex. 2014. PMID: 24763127 Free PMC article. - Banishing the Control Homunculi in Studies of Action Control and Behavior Change.
Verbruggen F, McLaren IP, Chambers CD. Verbruggen F, et al. Perspect Psychol Sci. 2014 Sep;9(5):497-524. doi: 10.1177/1745691614526414. Perspect Psychol Sci. 2014. PMID: 25419227 Free PMC article.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. - PubMed
- Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17:1426–1431. - PubMed
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reachiing movements to a visual target. Nat Neurosci. 1999;2:563–567. - PubMed
Publication types
MeSH terms
Grants and funding
- G0700399/MRC_/Medical Research Council/United Kingdom
- G0802146/MRC_/Medical Research Council/United Kingdom
- G0802146(89549)/MRC_/Medical Research Council/United Kingdom
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources