Role of cAMP/PKA signaling cascade in vasopressin-induced trafficking of TRPC3 channels in principal cells of the collecting duct - PubMed (original) (raw)
Role of cAMP/PKA signaling cascade in vasopressin-induced trafficking of TRPC3 channels in principal cells of the collecting duct
Monu Goel et al. Am J Physiol Renal Physiol. 2010 Apr.
Abstract
Transient receptor potential channels TRPC3 and TRPC6 are expressed in principal cells of the collecting duct (CD) along with the water channel aquaporin-2 (AQP2) both in vivo and in the cultured mouse CD cell line IMCD-3. The channels are primarily localized to intracellular vesicles, but upon stimulation with the antidiuretic hormone arginine vasopressin (AVP), TRPC3 and AQP2 translocate to the apical membrane. In the present study, the effect of various activators and inhibitors of the adenylyl cyclase (AC)/cAMP/PKA signaling cascade on channel trafficking was examined using immunohistochemical techniques and by biotinylation of surface membrane proteins. Both in vivo in rat kidney and in IMCD-3 cells, translocation of AQP2 and TRPC3 (but not TRPC6) was stimulated by [deamino-Cys(1), d-Arg(8)]-vasopressin (dDAVP), a specific V2-receptor agonist, and blocked by [adamantaneacetyl(1), O-Et-d-Tyr(2), Val(4), aminobutyryl(6), Arg(8,9)]-vasopressin (AEAVP), a specific V2-receptor antagonist. In IMCD-3 cells, translocation of TRPC3 and AQP2 was activated by forskolin, a direct activator of AC, or by dibutyryl-cAMP, a membrane-permeable cAMP analog. AVP-, dDAVP-, and forskolin-induced translocation in IMCD-3 cells was blocked by SQ22536 and H89, specific inhibitors of AC and PKA, respectively. Translocation stimulated by dibutyryl-cAMP was unaffected by AEAVP but could be blocked by H89. AVP- and forskolin-induced translocation of TRPC3 in IMCD-3 cells was also blocked by two additional inhibitors of PKA, specifically Rp-cAMPS and the myristoylated inhibitor of PKA (m-PKI). Quantification of TRPC3 membrane insertion in IMCD-3 cells under each assay condition using a surface membrane biotinylation assay, confirmed the translocation results observed by immunofluorescence. Importantly, AVP-induced translocation of TRPC3 as estimated by biotinylation was blocked on average 95.2 +/- 1.0% by H89, Rp-cAMPS, or m-PKI. Taken together, these results demonstrate that AVP stimulation of V2 receptors in principal cells of the CD causes translocation of TRPC3 to the apical membrane via stimulation of the AC/cAMP/PKA signaling cascade.
Figures
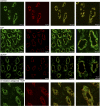
Fig. 1.
Effect of arginine vasopressin (AVP) and [deamino-Cys1,
d
-Arg8]-vasopressin (dDAVP) on the subcellular distribution of transient receptor potential channel TRPC3 and aquaporin-2 (AQP2) in medullary collecting duct of rat kidney. Anesthetized rats were injected with either saline control or saline containing AVP (25 ng), dDAVP (50 ng), or [adamantaneacetyl1, O-Et-
d
-Tyr2, Val4, Aminobutyryl6, Arg8,9]-vasopressin (AEAVP; 60 ng) followed by AVP (25 ng). The kidneys were removed after 30 min and processed for immunohistochemical analysis using fluorescence confocal microscopy as described in
methods
. Thin sections were colabeled with primary antibodies against TRPC3 (green) and AQP2 (red). The panels on the far right show selected merged images at higher magnification. In this and all subsequent figures, the results shown are representative of at least 3 independent experiments.
Fig. 2.
Effect of AVP and dDAVP on the subcellular distribution of TRPC6 and AQP2 in medullary collecting duct of rat kidney. The protocol was identical to that described in the legend to Fig 1. Thin sections were colabeled with primary antibodies against TRPC6 (green) and AQP2 (red). The far right panels show selected merged images at higher magnification.
Fig. 3.
Effect of AVP, dDAVP, and forskolin on the subcellular distribution of TRPC3 and AQP2 in inner medullary collecting duct (IMCD-3) cells. IMCD-3 cells were grown on glass coverslips until confluent. The monolayers were fixed and labeled with primary antibodies against TRPC3 (top, green) or TRPC6 (bottom, green) and AQP2 (red). The figure shows representative merged confocal images taken before (control) and 30 min after addition of AVP (50 nM), dDAVP (100 nM), or forskolin (100 μM) to the bath solution at 37°C.
Fig. 4.
Effect of the V2-antagonist AEAVP on AVP- and dDAVP-stimulated translocation of TRPC3 and AQP2 in IMCD-3 cells. The protocol was identical to that described in the legend to Fig 3. IMCD-3 cells were pretreated with AEAVP (100 nM) for 30 min before addition of AVP, dDAVP, or forskolin to the bath solution. The cell monolayers were fixed and labeled with primary antibodies against TRPC3 (green) and AQP2 (red). The figure shows representative merged confocal images of cells with and without pretreatment with AEAVP as indicated.
Fig. 5.
Effect of SQ22536 and H89 on AVP-, dDAVP-, and forskolin-stimulated translocation of TRPC3 and AQP2 in IMCD-3 cells. The protocol was identical to that described in the legend to Fig 3. IMCD-3 cells were pretreated with SQ22536 (100 μM), a specific inhibitor of adenylyl cyclase or H89 (1 μM), a specific inhibitor of PKA, for 30 min before addition of AVP, dDAVP, or forskolin to the bath solution. The cell monolayers were fixed and labeled with primary antibodies against TRPC3 (green) and AQP2 (red). The figure shows representative merged confocal images taken 30 min after addition of AVP, dDAVP, or forskolin as indicated.
Fig. 6.
Effect of dibutyryl-cAMP (db-cAMP) on TRPC3 and AQP2 translocation in IMCD-3 cells. IMCD-3 cells were pretreated with SQ22536 (100 μM) or H89 (1 μM) for 30 min before addition of dibutyryl-cAMP (100 μM) to the bath solution. The cell monolayers were fixed and labeled with primary antibodies against TRPC3 (green) and AQP2 (red). The figure shows representative merged confocal images taken 30 min after addition of dibutyryl-cAMP.
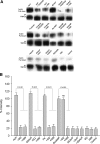
Fig. 7.
Biotinylation of plasmalemmal-associated TRPC3 in IMCD-3 cells treated with activators and inhibitors of the adenylyl cyclase (AC)/cAMP/PKA signaling cascade. A: surface membrane proteins were biotinylated in control IMCD-3 cells or in cells treated with the agents indicated above each lane. Whole cell lysates were subjected to avidin pulldown using streptavidin-agarose beads or immunoprecipitation (IP) using anti-TRPC3 antibodies as indicated to the left of each lane. The recovered proteins were separated by SDS-PAGE and analyzed by Western blotting for TRPC3 as described in
methods
. For each condition, the top avidin-pulldown band and the bottom TRPC3 IP band are from the same lysate and were run on the same gel. B: bands from 3 independent experiments were quantified by densitometry. The intensity of the TRPC3 bands from the avidin pulldowns were normalized to the value obtained for the corresponding TRPC3 IP bands under each condition.
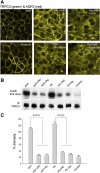
Fig. 8.
Effect of PKA inhibitors Rp-cAMPS and myristoylated inhibitor of PKA (m-PKI) on TRPC3 translocation. A: protocol was identical to that described in the legend to Fig 3. IMCD-3 cells were pretreated with Rp-cAMPs (10 μM) or m-PKI (1 μM) for 2 h before addition of AVP or forskolin to the bath solution. The cell monolayers were fixed and labeled with primary antibodies against TRPC3 (green) and AQP2 (red). The figure shows representative merged confocal images taken 30 min after addition of AVP or forskolin as indicated. B: surface biotinylation assays were performed as described in the legend to Fig 7 in control IMCD-3 cells or in cells pretreated with the PKA inhibitors as indicated above each lane. C: bands from 3 independent experiments were quantified by densitometry. The intensity of the TRPC3 bands from the avidin pulldowns were normalized to the value obtained for the corresponding TRPC3 IP bands under each condition.
Similar articles
- Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct.
Goel M, Sinkins WG, Zuo CD, Hopfer U, Schilling WP. Goel M, et al. Am J Physiol Renal Physiol. 2007 Nov;293(5):F1476-88. doi: 10.1152/ajprenal.00186.2007. Epub 2007 Aug 15. Am J Physiol Renal Physiol. 2007. PMID: 17699554 - Psychotropic drugs upregulate aquaporin-2 via vasopressin-2 receptor/cAMP/protein kinase A signaling in inner medullary collecting duct cells.
Kim S, Jo CH, Kim GH. Kim S, et al. Am J Physiol Renal Physiol. 2021 May 1;320(5):F963-F971. doi: 10.1152/ajprenal.00576.2020. Epub 2021 Apr 12. Am J Physiol Renal Physiol. 2021. PMID: 33843270 - Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking.
Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Li C, et al. Am J Physiol Renal Physiol. 2011 May;300(5):F1255-61. doi: 10.1152/ajprenal.00469.2010. Epub 2011 Feb 16. Am J Physiol Renal Physiol. 2011. PMID: 21325494 Free PMC article. - Modulation of vasopressin-elicited water transport by trafficking of aquaporin2-containing vesicles.
Ward DT, Hammond TG, Harris HW. Ward DT, et al. Annu Rev Physiol. 1999;61:683-97. doi: 10.1146/annurev.physiol.61.1.683. Annu Rev Physiol. 1999. PMID: 10099706 Review. - Role and identification of protein kinase A anchoring proteins in vasopressin-mediated aquaporin-2 translocation.
Klussmann E, Rosenthal W. Klussmann E, et al. Kidney Int. 2001 Aug;60(2):446-9. doi: 10.1046/j.1523-1755.2001.060002446.x. Kidney Int. 2001. PMID: 11473624 Review.
Cited by
- Evidence of a Role for Fibroblast Transient Receptor Potential Canonical 3 Ca2+ Channel in Renal Fibrosis.
Saliba Y, Karam R, Smayra V, Aftimos G, Abramowitz J, Birnbaumer L, Farès N. Saliba Y, et al. J Am Soc Nephrol. 2015 Aug;26(8):1855-76. doi: 10.1681/ASN.2014010065. Epub 2014 Dec 5. J Am Soc Nephrol. 2015. PMID: 25479966 Free PMC article. - Vasopressin regulates the growth of the biliary epithelium in polycystic liver disease.
Mancinelli R, Franchitto A, Glaser S, Vetuschi A, Venter J, Sferra R, Pannarale L, Olivero F, Carpino G, Alpini G, Onori P, Gaudio E. Mancinelli R, et al. Lab Invest. 2016 Nov;96(11):1147-1155. doi: 10.1038/labinvest.2016.93. Epub 2016 Aug 29. Lab Invest. 2016. PMID: 27571215 Free PMC article. - Acquired contractile ability in human endometrial stromal cells by passive loading of cyclic tensile stretch.
Kim J, Ushida T, Montagne K, Hirota Y, Yoshino O, Hiraoka T, Osuga Y, Furuakwa KS. Kim J, et al. Sci Rep. 2020 Jun 2;10(1):9014. doi: 10.1038/s41598-020-65884-3. Sci Rep. 2020. PMID: 32488068 Free PMC article. - Canonical Transient Receptor Potential 6 Channel: A New Target of Reactive Oxygen Species in Renal Physiology and Pathology.
Ma R, Chaudhari S, Li W. Ma R, et al. Antioxid Redox Signal. 2016 Nov 1;25(13):732-748. doi: 10.1089/ars.2016.6661. Epub 2016 Mar 18. Antioxid Redox Signal. 2016. PMID: 26937558 Free PMC article. Review. - Calcium signalling and transport in the kidney.
Staruschenko A, Alexander RT, Caplan MJ, Ilatovskaya DV. Staruschenko A, et al. Nat Rev Nephrol. 2024 Aug;20(8):541-555. doi: 10.1038/s41581-024-00835-z. Epub 2024 Apr 19. Nat Rev Nephrol. 2024. PMID: 38641658 Review.
References
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6: 709–720, 2004 - PubMed
- Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003. - PubMed
- Brown D, Nielsen S. The cell biology of vasopressin action. In: The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders, 2004
- Cayouette S, Boulay G. Intracellular trafficking of TRP channels. Cell Calcium 42: 225–232, 2007 - PubMed
- Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq-protein-coupled receptor activation. J Biol Chem 279: 7241–7246, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous