A novel 3-D mineralized tumor model to study breast cancer bone metastasis - PubMed (original) (raw)
A novel 3-D mineralized tumor model to study breast cancer bone metastasis
Siddharth P Pathi et al. PLoS One. 2010.
Abstract
Background: Metastatic bone disease is a frequent cause of morbidity in patients with advanced breast cancer, but the role of the bone mineral hydroxyapatite (HA) in this process remains unclear. We have developed a novel mineralized 3-D tumor model and have employed this culture system to systematically investigate the pro-metastatic role of HA under physiologically relevant conditions in vitro.
Methodology/principal findings: MDA-MB231 breast cancer cells were cultured within non-mineralized or mineralized polymeric scaffolds fabricated by a gas foaming-particulate leaching technique. Tumor cell adhesion, proliferation, and secretion of pro-osteoclastic interleukin-8 (IL-8) was increased in mineralized tumor models as compared to non-mineralized tumor models, and IL-8 secretion was more pronounced for bone-specific MDA-MB231 subpopulations relative to lung-specific breast cancer cells. These differences were pathologically significant as conditioned media collected from mineralized tumor models promoted osteoclastogenesis in an IL-8 dependent manner. Finally, drug testing and signaling studies with transforming growth factor beta (TGFbeta) confirmed the clinical relevance of our culture system and revealed that breast cancer cell behavior is broadly affected by HA.
Conclusions/significance: Our results indicate that HA promotes features associated with the neoplastic and metastatic growth of breast carcinoma cells in bone and that IL-8 may play an important role in this process. The developed mineralized tumor models may help to reveal the underlying cellular and molecular mechanisms that may ultimately enable more efficacious therapy of patients with advanced breast cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
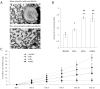
Figure 1. Physicochemical characterization of scaffolds.
(A) HA in biomineralized scaffolds is available for cellular interactions as EDS analysis indicates Ca and P at the porous surface of the scaffolds, while no mineral was detected for non-mineralized control scaffolds. (B) MicroCT scans indicate that HA is uniformly distributed throughout biomineralized scaffolds, but was not present non-mineralized control scaffolds. (C) Incorporation of HA did not alter the scaffold microarchitecture relative to non-mineralized control scaffolds as indicated by visualization via brightfield microscopy. Scale bars represent 2 mm. (D) Image analysis of high-resolution brightfield microscopy images indicates that pore size and polymer wall thickness are similar for both biomineralized and non-mineralized scaffolds.
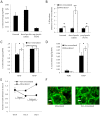
Figure 2. Effect of HA on breast cancer cell adhesion.
(A) Analysis of von Kossa stained histological cross-sections indicates that MDA-MB231 cells (stained red) penetrate into the center of mineralized scaffolds (HA stained black), while this is not the case for non-mineralized control scaffolds. Arrows and asterisks indicate representative scaffold walls and pores, respectively. Scale bars represent 200 µm. (B) MDA-MB231 breast cancer cells adhere more efficiently to mineralized scaffolds than non-mineralized scaffolds (**p<0.01), and pre-incubation of MDA-MB231 cells with RGD peptide inhibits enhanced adhesion to mineralized scaffolds (**p<0.01). Error bars are small where not visible. (C) Fibronectin adsorption within the polymer scaffold is increased in mineralized scaffolds (lane 2) relative to non-mineralized scaffolds (lane 1) as indicated by Western Blot analysis of scaffold lysates.
Figure 3. Effect of HA on 3-D tumor tissue formation.
(A) Quantification of DNA indicates enhanced proliferation of MDA-MB231 cells within mineralized scaffolds as compared to non-mineralized scaffolds. (B) Analysis of von Kossa stained histological cross-sections revealed that 5 days after seeding, coherent tissue begins to form in mineralized scaffolds but not non-mineralized scaffolds (MDA-MB-231 cells stained red, HA stained black). Scale bars represent 50 µm. (C) Live and dead staining with calcein (green) and propidium iodide (red), respectively, shows increased cell number and tissue formation into pores of mineralized scaffolds relative to control scaffolds. White scale bars represent 100 µm.
Figure 4. Osteoclastogenesis in response to conditioned media.
(A) Conditioned media collected from mineralized tumor models increased RAW 264.7 osteoclastogenesis relative to conditioned media collected from non-mineralized tumor models as revealed by TRAP staining of large multinucleated cells. (B) Quantification of TRAP+ cells indicated that culture media collected from mineralized scaffold cultures (MIN+) promoted RAW 264.7 osteoclastogenesis relative to control media (cDMEM) and media collected from non-mineralized scaffold cultures (MIN-) in a manner that was similar to osteoclastic RANKL. Asterisks [*p<0.05, **p<0.01] and pound signs [#p<0.05, ##p<0.01] indicate statistical significance with respect to ‘cDMEM’ and ‘MIN-’, respectively. (C) Conditioned media collected from mineralized models (MIN+) enhances the resorptive activity of RAW 264.7 relative to all other tested conditions as indicated by 2-D culture on calcium phosphate disks and subsequent analysis of calcium release by a colorimetric assay.
Figure 5. Osteoclastogenesis in response to HA-dependent IL-8 signaling.
(A) Tumor cells cultured within mineralized scaffolds up-regulated secretion of IL-8 relative to culture within non-mineralized control scaffolds, while no effect was detected for VEGF and IL-11 secretion (*p<0.05). Error bars for IL-11 are large due to low IL-11 secretion. (B) Blockade of IL-8 signaling by addition of a neutralizing antibody inhibited the pro-osteoclastic effect of conditioned media collected from mineralized tumors (MIN+) to levels comparable to non-mineralized cultures (MIN-) (*p<0.05). (C) Transwell assays with conditioned media indicate that tumor cells cultured within mineralized scaffolds (MIN+) increase the motility of RAW 264.7 relative to all other conditions. Inhibition with a function blocking antibody suggested that this effect was IL-8 dependent. (D) Colorimetric analysis of Ca-release indicates that IL-8 neutralization in media collected from mineralized tumor models (Ab/MIN+) results in a much more pronounced decrease in osteoclast activity as compared to media collected from non-mineralized scaffold cultures (Ab/MIN-). (E) Micrographs of osteoclast-mediated pit formation on bone mineral surface in the presence of conditioned media from mineralized tumor models with and without IL-8 antibody. Scale bars represent 200 µm. (F) Quantification of pit formation in response to the different media in the presence and absence of functional IL-8 signaling.
Figure 6. Clinical relevance of 3-D mineralized tumor models.
(A) 1833 bone-metastatic MDA-MB231 cells secrete more IL-8 than parental and 4175 lung-metastatic cells in 2-D culture. (B) Mineralized scaffold culture increases IL-8 secretion for all MDA-MB231 populations, but this response is significantly increased in bone-metastatic cells as compared to parental and lung-metastatic cells (**p<0.01, p<0.05). (C) TGFβ1 up-regulates IL-8 secretion in non-mineralized, but not mineralized 3-D cultures of MDA-MB231. (D) In contrast, TGFβ1 increases IL-11 secretion more significantly in mineralized than non-mineralized cultures (p<0.05). (E) Tumor cells cultured within mineralized scaffolds exhibit increased growth suppression in response to ibandronate both when directly exposed to the drug in the dosed interval (days 1–3) and after the drug was removed during the undosed interval (days 3–5). (F) Live/dead staining with calcein (green) and propidium iodide (red) confirmed increased cell death in response to ibandronate in mineralized cultures relative to non-mineralized cultures. Scale bars represent 100 µm.
Similar articles
- Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis.
Pathi SP, Lin DD, Dorvee JR, Estroff LA, Fischbach C. Pathi SP, et al. Biomaterials. 2011 Aug;32(22):5112-22. doi: 10.1016/j.biomaterials.2011.03.055. Epub 2011 Apr 20. Biomaterials. 2011. PMID: 21507478 Free PMC article. - Multiscale characterization of the mineral phase at skeletal sites of breast cancer metastasis.
He F, Chiou AE, Loh HC, Lynch M, Seo BR, Song YH, Lee MJ, Hoerth R, Bortel EL, Willie BM, Duda GN, Estroff LA, Masic A, Wagermaier W, Fratzl P, Fischbach C. He F, et al. Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):10542-10547. doi: 10.1073/pnas.1708161114. Epub 2017 Sep 18. Proc Natl Acad Sci U S A. 2017. PMID: 28923958 Free PMC article. - Intrafibrillar, bone-mimetic collagen mineralization regulates breast cancer cell adhesion and migration.
Choi S, Friedrichs J, Song YH, Werner C, Estroff LA, Fischbach C. Choi S, et al. Biomaterials. 2019 Apr;198:95-106. doi: 10.1016/j.biomaterials.2018.05.002. Epub 2018 May 7. Biomaterials. 2019. PMID: 29759731 Free PMC article. - Parathyroid hormone-related protein and bone metastases.
Guise TA. Guise TA. Cancer. 1997 Oct 15;80(8 Suppl):1572-80. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. Cancer. 1997. PMID: 9362424 Review. - Breast cancer-derived factors facilitate osteolytic bone metastasis.
Rose AA, Siegel PM. Rose AA, et al. Bull Cancer. 2006 Sep;93(9):931-43. Bull Cancer. 2006. PMID: 16980236 Review.
Cited by
- State of the Art Modelling of the Breast Cancer Metastatic Microenvironment: Where Are We?
Nuckhir M, Withey D, Cabral S, Harrison H, Clarke RB. Nuckhir M, et al. J Mammary Gland Biol Neoplasia. 2024 Jul 16;29(1):14. doi: 10.1007/s10911-024-09567-z. J Mammary Gland Biol Neoplasia. 2024. PMID: 39012440 Free PMC article. Review. - Current Advances in the Use of Tissue Engineering for Cancer Metastasis Therapeutics.
Katti PD, Jasuja H. Katti PD, et al. Polymers (Basel). 2024 Feb 23;16(5):617. doi: 10.3390/polym16050617. Polymers (Basel). 2024. PMID: 38475301 Free PMC article. Review. - Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix.
Ditto M, Jacho D, Eisenmann KM, Yildirim-Ayan E. Ditto M, et al. Bioengineering (Basel). 2023 Oct 31;10(11):1271. doi: 10.3390/bioengineering10111271. Bioengineering (Basel). 2023. PMID: 38002395 Free PMC article. - PI3K/AKT signaling activates HIF1α to modulate the biological effects of invasive breast cancer with microcalcification.
Tian Y, Zhao L, Gui Z, Liu S, Liu C, Yu T, Zhang L. Tian Y, et al. NPJ Breast Cancer. 2023 Nov 13;9(1):93. doi: 10.1038/s41523-023-00598-z. NPJ Breast Cancer. 2023. PMID: 37957150 Free PMC article. - Bone-homing metastatic breast cancer cells impair osteocytes' mechanoresponse in a 3D loading model.
Sarazin BA, Liu B, Goldman E, Whitefield AN, Lynch ME. Sarazin BA, et al. Heliyon. 2023 Sep 21;9(10):e20248. doi: 10.1016/j.heliyon.2023.e20248. eCollection 2023 Oct. Heliyon. 2023. PMID: 37767467 Free PMC article.
References
- Guise TA. Antitumor effects of bisphosphonates: promising preclinical evidence. Cancer Treat Rev. 2008;34(Suppl 1):S19–24. - PubMed
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. - PubMed
- Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. - PubMed
- Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical