Coexistence of quiescent and active adult stem cells in mammals - PubMed (original) (raw)
Review
Coexistence of quiescent and active adult stem cells in mammals
Linheng Li et al. Science. 2010.
Abstract
Adult stem cells are crucial for physiological tissue renewal and regeneration after injury. Prevailing models assume the existence of a single quiescent population of stem cells residing in a specialized niche of a given tissue. Emerging evidence indicates that both quiescent (out of cell cycle and in a lower metabolic state) and active (in cell cycle and not able to retain DNA labels) stem cell subpopulations may coexist in several tissues, in separate yet adjoining locations. Here, we summarize these findings and propose that quiescent and active stem cell populations have separate but cooperative functional roles.
Figures
Fig. 1
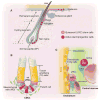
Stem cell locations in hair follicle, gut, and bone marrow. (A) Hair follicle structure with quiescent (bulge) and active (hair germ) stem/progenitor cells. Bulge area typically maintains quiescent stem cells, whereas DP provides stimulatory signals. Only during development and under injury condition, bulge stem cells give rise to stem cells in epidermis. (B) Intestinal crypt structure with quiescent (+4) and active CBC (Lgr5+) stem cells, as well as TA and mesenchymal cells. (C) Quiescent HSCs located in the endosteal region where osteoblastic lining, endothelial, CAR, and other cells form the endosteal region and active HSCs located in the central marrow region, which lacks osteoblastic cells.
Fig. 2
DP position determines state of stem/progenitor cells in hair follicle. (A) Hair cycle. At the start of anagen, DP is proximal to hair germ and bulge. Stem/progenitor cells in hair germ (a structure below the bulge) are activated by DP first, whereas stem cells remain quiescent in bulge. When hair germ cells enter the hair matrix, then stem cells in bulge are activated to replenish lost hair germ cells. During anagen, active hair germ stem cells give rise to TA cells, which in turn support hair growth. DP is pushed down from bulge because of fast expansion of progenitor cells. In catagen, DP retracts toward bulge. (B) Co-staining of BrdU with phosphorylated-Akt shows relationship between quiescent stem cells (yellow arrow) and active stem cells (red arrows) in bulge and hair germ. [Reprinted by permission from John Wiley and Sons, Incorporated, AlphaMed Press, p. 2834 of (20).] (C and D ) CD34+Lgr5− and CD34+Lgr5+ stem cells, respectively, located inside and next to bulge area (C), which is consistent with Lgr5+ stem cells located adjacent to LRCs (D). [Reprinted by permission from Macmillan Publishers, Limited, pp. 1292 and 1293 of (19).]
Fig. 3
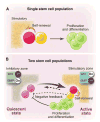
Two models for stem cell-based tissue self-renewal and regeneration. (A) Prevailing model of a single stem cell population located in the niche. Asymmetric division is the key mechanism to maintain balance between self-renewal and differentiation. (B) Proposed alternative and complementary model: co-existing quiescent and active stem cell populations located in adjacent zones with corresponding inhibitory and stimulatory signals. Quiescent stem cells replace damaged active stem cells (heavy arrow). Conversely, active stem cells may replace lost quiescent stem cells (light arrow). A negative feedback from either active stem cells (dashed line) or their progeny (solid line) may contribute to prevention of quiescent stem cells from activation.
Fig. 4
Zoned stem cell population and the associated microenvironmental signals. (A) Bulge area typically provides Wnt-off and BMP-on signals thus maintaining quiescent stem cells, whereas DP provides stimulatory (Wnt-on and BMP-off) signals. (B) Similarly, in intestinal crypt (+4) ISCs are maintained by Wnt-off and BMP-on signals. In contrast, CBC (Lgr5+) stem cells are exposed to Wnt-on but BMP-off signals. (C) Quiescent HSCs located in the endosteal zone where osteoblastic lining cells provide dominant inhibitory signals including BMP, OPN, and sFRP1. In contrast, HSCs located in the central marrow zone are stimulated by endothelial, megakaryocyte, and CAR cells secreting Wnt, FGF, and SDF1.
Similar articles
- Compartmentalized organization: a common and required feature of stem cell niches?
Greco V, Guo S. Greco V, et al. Development. 2010 May;137(10):1586-94. doi: 10.1242/dev.041103. Development. 2010. PMID: 20430743 Free PMC article. Review. - Molecular Regulation of Cellular Quiescence: A Perspective from Adult Stem Cells and Its Niches.
So WK, Cheung TH. So WK, et al. Methods Mol Biol. 2018;1686:1-25. doi: 10.1007/978-1-4939-7371-2_1. Methods Mol Biol. 2018. PMID: 29030809 Review. - Two anatomically distinct niches regulate stem cell activity.
Ema H, Suda T. Ema H, et al. Blood. 2012 Sep 13;120(11):2174-81. doi: 10.1182/blood-2012-04-424507. Epub 2012 Jul 11. Blood. 2012. PMID: 22786878 Review. - Dormancy in the stem cell niche.
Sottocornola R, Lo Celso C. Sottocornola R, et al. Stem Cell Res Ther. 2012 Mar 19;3(2):10. doi: 10.1186/scrt101. Stem Cell Res Ther. 2012. PMID: 22429750 Free PMC article. Review. - Stem cells and their niches.
Moore KA, Lemischka IR. Moore KA, et al. Science. 2006 Mar 31;311(5769):1880-5. doi: 10.1126/science.1110542. Science. 2006. PMID: 16574858 Review.
Cited by
- Ageing-associated long non-coding RNA extends lifespan and reduces translation in non-dividing cells.
Anver S, Sumit AF, Sun XM, Hatimy A, Thalassinos K, Marguerat S, Alic N, Bähler J. Anver S, et al. EMBO Rep. 2024 Oct 2. doi: 10.1038/s44319-024-00265-9. Online ahead of print. EMBO Rep. 2024. PMID: 39358553 - Chromatin remodeling in tissue stem cell fate determination.
Li X, Zhu G, Zhao B. Li X, et al. Cell Regen. 2024 Sep 30;13(1):18. doi: 10.1186/s13619-024-00203-z. Cell Regen. 2024. PMID: 39348027 Free PMC article. Review. - Establishment of a 3D organoid culture model for the investigation of adult slow-cycling putative intestinal stem cells.
Gulino ME, Ordóñez-Morán P, Mahida YR. Gulino ME, et al. Histochem Cell Biol. 2024 Nov;162(5):351-362. doi: 10.1007/s00418-024-02312-x. Epub 2024 Jul 29. Histochem Cell Biol. 2024. PMID: 39073425 - The Principle of Cortical Development and Evolution.
Yang Z. Yang Z. Neurosci Bull. 2024 Jul 18. doi: 10.1007/s12264-024-01259-2. Online ahead of print. Neurosci Bull. 2024. PMID: 39023844 Review. - The miRNA Contribution in Adipocyte Maturation.
Giammona A, Di Franco S, Lo Dico A, Stassi G. Giammona A, et al. Noncoding RNA. 2024 Jun 12;10(3):35. doi: 10.3390/ncrna10030035. Noncoding RNA. 2024. PMID: 38921832 Free PMC article.
References
- Weissman IL. Cell. 2000;100:157. - PubMed
- Kimble JE, White JG. Dev Biol. 1981;81:208. - PubMed
- Spradling A, Drummond-Barbosa D, Kai T. Nature. 2001;414:98. - PubMed
- Song X, Call GB, Kirilly D, Xie T. Development. 2007;134:1071. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 DK085507/DK/NIDDK NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- U01DK085507/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources