Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N-oxide - PubMed (original) (raw)
Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N-oxide
Gabriel Mitchell et al. BMC Microbiol. 2010.
Abstract
Background: Staphylococcus aureus and Pseudomonas aeruginosa are often found together in the airways of cystic fibrosis (CF) patients. It was previously shown that the P. aeruginosa exoproduct 4-hydroxy-2-heptylquinoline-N-oxide (HQNO) suppresses the growth of S. aureus and provokes the emergence of small-colony variants (SCVs). The presence of S. aureus SCVs as well as biofilms have both been associated with chronic infections in CF.
Results: We demonstrated that HQNO stimulates S. aureus to form a biofilm in association with the formation of SCVs. The emergence of SCVs and biofilm production under HQNO exposure was shown to be dependent on the activity of the stress- and colonization-related alternative sigma factor B (SigB). Analysis of gene expression revealed that exposure of a prototypical S. aureus strain to HQNO activates SigB, which was leading to an increase in the expression of the fibronectin-binding protein A and the biofilm-associated sarA genes. Conversely, the quorum sensing accessory gene regulator (agr) system and the alpha-hemolysin gene were repressed by HQNO. Experiments using culture supernatants from P. aeruginosa PAO1 and a double chamber co-culture model confirmed that P. aeruginosa stimulates biofilm formation and activates SigB in a S. aureus strain isolated from a CF patient. Furthermore, the supernatant from P. aeruginosa mutants unable to produce HQNO induced the production of biofilms by S. aureus to a lesser extent than the wild-type strain only in a S. aureus SigB-functional background.
Conclusions: These results suggest that S. aureus responds to HQNO from P. aeruginosa by forming SCVs and biofilms through SigB activation, a phenomenon that may contribute to the establishment of chronic infections in CF patients.
Figures
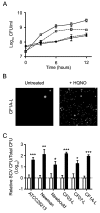
Figure 1
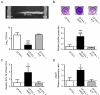
HQNO inhibits the growth of normal S. aureus strains and provokes the emergence of SCVs. (A) Growth curves of the normal strain CF1A-L (□) and the SCV CF1D-S (●) exposed (dotted lines) or not (solid lines) to 10 μg/ml of HQNO. (B) Pictures show SCV colonies grown on agar containing a selective concentration of gentamicin following or not an overnight treatment of strain CF1A-L with 10 μg/ml of HQNO. (C) Relative number of SCV CFUs recovered after 18 h of growth from strains ATCC 29213, Newman, Newbould, CF03-L, CF07-L and CF1A-L following (black bars) or not (open bars) treatments with 10 μg/ml of HQNO. Data are presented as means with standard deviations from at least three independent experiments. Results are normalized to the non exposed condition for each strain (dotted line). Significant differences between untreated and HQNO-treated conditions are shown (*, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired _t-_test).
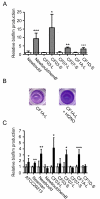
Figure 2
HQNO stimulates biofilm production in normal strains but does not alter high biofilm production in SCVs. (A) Relative biofilm production in related normal (open bars) and SCV (grey bars) strains. Results are normalized to the normal strain for each pair (dotted line). (B) Pictures show the biofilm formation of the normal strain CF1A-L in the absence or in the presence of HQNO as detected by crystal violet staining. (C) Relative biofilm production in strains exposed (black bars) or not (open bars) to 10 μg/ml of HQNO. Results are normalized to the unexposed condition for each strain (dotted line). Data are presented as means with standard deviations from at least three independent experiments. Significant differences between normal and SCV strains (-L and -S suffixes, respectively) or between unexposed and HQNO-exposed conditions are shown (*, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired _t-_test).
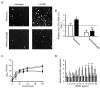
Figure 3
SigB is involved in HQNO-mediated emergence of SCVs and biofilm production. (A) Pictures show SCV colonies grown on agar containing a selective concentration of gentamicin following or not an overnight exposure to 10 μg/ml of HQNO for strains Newbould and NewbouldΔ_sigB_. (B) Relative number of SCV CFUs recovered after 18 h of growth for strains Newbould and NewbouldΔ_sigB_ in the presence (black bars) or not (open bars) of 10 μg HQNO/ml. Results are normalized to unexposed Newbould (dotted line). Data are presented as means with standard deviations from at least three independent experiments. Significant differences between unexposed and HQNO-exposed conditions (*, P < 0.05), and between strains in the same experimental condition (Δ, P < 0.05) were revealed by a one-way ANOVA with tuckey's post test. (C) Growth curves of Newbould (□) and NewbouldΔsigB (●) exposed (dotted lines) or not (solid lines) to 10 μg/ml of HQNO. (D) Relative biofilm formation as a function of the concentration of HQNO for strains Newbould (open bars) and NewbouldΔ_sigB_ (grey bars). Results are normalized to the unexposed condition for each strain (dotted line). Data are presented as means with standard deviations from two independent experiments. Significant differences between Newbould and NewbouldΔ_sigB_ for each concentration of HQNO are shown (*, P < 0.05; **, P < 0.01; two-way ANOVA with bonferroni's post test).
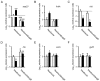
Figure 4
SigB and agr activities are modulated by an exposure to HQNO. Relative expression ratios for the genes asp23 (A), fnbA (B), hld (C), hla (D), sarA (E) and gyrB (F) were evaluated by qPCR for strains Newbould and NewbouldΔ_sigB_ grown to the exponential phase in the presence (black bars) or in the absence (open bars) of 10 μg/ml of HQNO. Results are normalized to unexposed Newbould (dotted line). Data are presented as means with standard deviations from at least three independent experiments. Significant differences between the unexposed and HQNO-exposed conditions (*, P < 0.05; ***, P < 0.001) and between Newbould and NewbouldΔ_sigB_ for the same experimental condition (Δ, P < 0.05; ΔΔ, P < 0.01; ΔΔΔ, P < 0.001) were revealed by one-way ANOVA followed by the tuckey's post test.
Figure 5
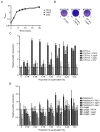
P. aeruginosa stimulates biofilm formation and increases the activity of SigB of a S. aureus CF isolate. (A) CFU/ml recovered after 48 h of growth of CF1A-L (open bar) and CF1A-L in the presence of supernatants from overnight cultures of P. aeruginosa PAO1 (black bar) or of E. coli K12 (hatched bar). The picture shows the specific inhibitory effect of P. aeruginosa on the growth of S. aureus. (B) Relative biofilm production by CF1A-L grown in the presence of supernatants from overnight cultures of P. aeruginosa or E. coli. Pictures show the biofilm formation of CF1A-L in the absence or in the presence of culture supernatants of P. aeruginosa or E. coli as detected by crystal violet staining. (C) Relative number of SCV CFUs recovered after 6 h of growth for S. aureus CF1A-L in co-culture with PAO1 or K12 as determined using the double chamber co-culture model. (D) Relative expression ratios for the gene asp23 were evaluated by qPCR for CF1A-L in co-culture with PAO1 or K12. For B, C and D, results are normalized to unexposed CF1A-L (dotted line). Data are presented as means with standard deviations from three independent experiments. Significant differences between unexposed CF1A-L and the exposed conditions (*, P < 0.05; ***, P < 0.001) and between CF1A-L exposed to PAO1 or K12 (Δ, P < 0.05; ΔΔΔ, P < 0.001) were revealed by one-way ANOVA followed by the tuckey's post test.
Figure 6
HQNO from P. aeruginosa stimulates biofilm production of S. aureus strains by a SigB-dependent mechanism. (A) Growth curves of P. aeruginosa strain PA14 and the pqsA and pqsL mutants. (B) Pictures show relative biofilm production of CF1A-L in the absence or in the presence of supernatants from overnight cultures of P. aeruginosa PA14 or the pqsL mutant as determined by crystal violet staining. (C) Relative biofilm production by S. aureus CF1A-L as a function of the proportion of supernatant from overnight cultures of P. aeruginosa PA14, the pqsA mutant, the pqsL mutant or E. coli K12. Results are normalized to unexposed CF1A-L (dotted line). Significant differences between CF1A-L+PA14 and the other conditions for each proportion of supernatant are shown (*, P < 0.05; two-way ANOVA with Bonferroni's post test). (D) Relative biofilm production by S. aureus strains Newbould and NewbouldΔ_sigB_ as a function of the proportion of supernatant from overnight cultures of P. aeruginosa PA14, the pqsA or the pqsL mutant. Significant differences between Newbould + PA14 and the other conditions for each proportion of supernatant (*, P < 0.05; two-way ANOVA with Bonferroni's post test), and between NewbouldΔ_sigB_ + PA14 and Newbould Δ_sigB_ + the pqsA or the pqsL mutant (Δ, P < 0.05; two-way ANOVA with Bonferroni's post test) are shown. The significant difference between untreated Newbould and NewbouldΔ_sigB_ is also shown (#, P < 0.05; unpaired _t_-test). Data are presented as means with standard deviations from at least three independent experiments.
Similar articles
- A role for sigma factor B in the emergence of Staphylococcus aureus small-colony variants and elevated biofilm production resulting from an exposure to aminoglycosides.
Mitchell G, Brouillette E, Séguin DL, Asselin AE, Jacob CL, Malouin F. Mitchell G, et al. Microb Pathog. 2010 Jan;48(1):18-27. doi: 10.1016/j.micpath.2009.10.003. Epub 2009 Oct 13. Microb Pathog. 2010. PMID: 19825410 - Pseudomonas aeruginosa Alters Staphylococcus aureus Sensitivity to Vancomycin in a Biofilm Model of Cystic Fibrosis Infection.
Orazi G, O'Toole GA. Orazi G, et al. mBio. 2017 Jul 18;8(4):e00873-17. doi: 10.1128/mBio.00873-17. mBio. 2017. PMID: 28720732 Free PMC article. - Interconnections between Sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation.
Lauderdale KJ, Boles BR, Cheung AL, Horswill AR. Lauderdale KJ, et al. Infect Immun. 2009 Apr;77(4):1623-35. doi: 10.1128/IAI.01036-08. Epub 2009 Feb 2. Infect Immun. 2009. PMID: 19188357 Free PMC article. - Biofilm and Small Colony Variants-An Update on Staphylococcus aureus Strategies toward Drug Resistance.
Guo H, Tong Y, Cheng J, Abbas Z, Li Z, Wang J, Zhou Y, Si D, Zhang R. Guo H, et al. Int J Mol Sci. 2022 Jan 22;23(3):1241. doi: 10.3390/ijms23031241. Int J Mol Sci. 2022. PMID: 35163165 Free PMC article. Review. - Pseudomonas aeruginosa and Staphylococcus aureus communication in biofilm infections: insights through network and database construction.
Magalhães AP, Jorge P, Pereira MO. Magalhães AP, et al. Crit Rev Microbiol. 2019 Sep-Nov;45(5-6):712-728. doi: 10.1080/1040841X.2019.1700209. Epub 2019 Dec 13. Crit Rev Microbiol. 2019. PMID: 31835971 Review.
Cited by
- SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants.
Mitchell G, Fugère A, Pépin Gaudreau K, Brouillette E, Frost EH, Cantin AM, Malouin F. Mitchell G, et al. PLoS One. 2013 May 21;8(5):e65018. doi: 10.1371/journal.pone.0065018. Print 2013. PLoS One. 2013. PMID: 23705029 Free PMC article. - Maintenance of S. aureus in Co-culture With P. aeruginosa While Growing as Biofilms.
Woods PW, Haynes ZM, Mina EG, Marques CNH. Woods PW, et al. Front Microbiol. 2019 Jan 9;9:3291. doi: 10.3389/fmicb.2018.03291. eCollection 2018. Front Microbiol. 2019. PMID: 30687276 Free PMC article. - Bacterial Interactions in the Context of Chronic Wound Biofilm: A Review.
Durand BARN, Pouget C, Magnan C, Molle V, Lavigne JP, Dunyach-Remy C. Durand BARN, et al. Microorganisms. 2022 Jul 25;10(8):1500. doi: 10.3390/microorganisms10081500. Microorganisms. 2022. PMID: 35893558 Free PMC article. Review. - Phenotypic and Genotypic Characteristics of Small Colony Variants and Their Role in Chronic Infection.
Johns BE, Purdy KJ, Tucker NP, Maddocks SE. Johns BE, et al. Microbiol Insights. 2015 Sep 22;8:15-23. doi: 10.4137/MBI.S25800. eCollection 2015. Microbiol Insights. 2015. PMID: 26448688 Free PMC article. Review. - Ecological networking of cystic fibrosis lung infections.
Quinn RA, Whiteson K, Lim YW, Zhao J, Conrad D, LiPuma JJ, Rohwer F, Widder S. Quinn RA, et al. NPJ Biofilms Microbiomes. 2016 Dec 2;2:4. doi: 10.1038/s41522-016-0002-1. eCollection 2016. NPJ Biofilms Microbiomes. 2016. PMID: 28649398 Free PMC article.
References
- Hoffman LR, Deziel E, D'Argenio DA, Lepine F, Emerson J, McNamara S, Gibson RL, Ramsey BW, Miller SI. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2006;103(52):19890–19895. doi: 10.1073/pnas.0606756104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources