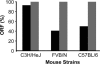Expression of the BabA adhesin during experimental infection with Helicobacter pylori - PubMed (original) (raw)
Expression of the BabA adhesin during experimental infection with Helicobacter pylori
Cathy M Styer et al. Infect Immun. 2010 Apr.
Abstract
The Helicobacter pylori babA gene encodes an outer membrane protein that mediates binding to fucosylated ABH antigens of the ABO blood group. We recently demonstrated that BabA expression is lost during experimental infection of rhesus macaques with H. pylori J166. We sought to test the generality of this observation by comparison of different H. pylori strains and different animal hosts. Challenge of macaques with H. pylori J99 yielded output strains that lost BabA expression, either by selection and then expansion of a subpopulation of J99 that had a single-base-pair mutation that encoded a stop codon or by gene conversion of babA with a duplicate copy of babB, a paralog of unknown function. Challenge of mice with H. pylori J166, which unlike J99, has 5' CT repeats in babA, resulted in loss of BabA expression due to phase variation. In the gerbil, Leb binding was lost by replacement of the babA gene that encoded Leb binding with a nonbinding allele that differed at six amino acid residues. Complementation experiments confirmed that change in these six amino acids of BabA was sufficient to eliminate binding to Leb and to gastric tissue. These results demonstrate that BabA expression in vivo is highly dynamic, and the findings implicate specific amino acid residues as critical for binding to fucosylated ABH antigens. We hypothesize that modification of BabA expression during H. pylori infection is a mechanism to adapt to changing conditions of inflammation and glycan expression at the epithelial surface.
Figures
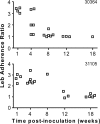
FIG. 1.
Leb adherence ratio for individual H. pylori colonies isolated from two rhesus macaques (30364 and 31105) 1 to 18 weeks after inoculation with strain H. pylori J99. H. pylori attachment to Leb decreased significantly with time after inoculation (Kruskal-Wallis test, two tailed, P < 0.001).
FIG. 2.
Percentage of H. pylori output colonies with an ORF for babA (▪) and babB (░⃞) 2 weeks postinoculation with strain J166 in three strains of mice (n = 4 to 6 mice/strain). Percentages were calculated as a fraction of total colonies (13 to 32 per strain) isolated from each mouse strain. An ORF was present if DNA sequencing demonstrated the presence of eight CT dinucleotide repeats in the 5′ end of babA. Out of frame sequences had seven or nine CT repeats, which resulted in a stop codon at bp 78 (addition of CT) or bp 48 (loss of CT).
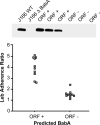
FIG. 3.
Verification of expression and Leb adherence in H. pylori J166 with a babA ORF. Immunoblot of representative single-colony isolates from mouse output strains with an in frame (ORF+) or out of frame (ORF−) babA gene probed with antibody to BabA (top panel). Wild-type H. pylori J166 (J166WT) and an isogenic babA knockout (J166r_babA_) served as controls. Only strains with the babA ORF expressed protein. Analysis of Leb binding (bottom panel) of H. pylori J166 single-colony mouse output strains showed that those with a babA ORF showed significantly greater attachment to Leb (Mann-Whitney U test, two tailed, P < 0.001).
FIG. 4.
Schematic representation of the gene conversion event that occurred in H. pylori strain 7.13 after 12 weeks of colonization in gerbils. Input strain 7.13 has an expressed BabA2 and a silent BabA1, which has one additional adenine residue in a polynucleotide tract (7A versus 6A) that begins immediately after the ATG start codon and results in a stop codon at bp 61. Shown are the 11 amino acid residues (single-letter designations numbered according to the mature protein after removal of the signal peptide) for BabA1 that differ from those predicted in BabA2 if the protein were expressed. Gerbil output strain 8.31 has undergone gene conversion of babA2 by babA1. The recombination occurred so as to retain the 6A polynucleotide tract, so BabA2 is expressed, but the replacement of 6 amino acids from BabA2 with those from BabA1 (shaded) is sufficient to eliminate binding to Leb.
FIG. 5.
Gastric histosections from gerbils that were uninfected (A to D) or infected (E to H) with H. pylori. Hematoxylin and eosin stained sections (A and E) demonstrate the marked cellular infiltrate and loss of normal epithelial architecture that is characteristic of H. pylori gastritis. Incubation of uninfected tissue sections with FITC-labeled bacteria showed that H. pylori 7.13, which binds Leb, also attached to gastric tissue (B), whereas strains 8.31 (C) and 7.13 expressing the 8.31 babA (D) did not. Interestingly, none of the strains bound appreciably to infected gerbil tissue (F to H), suggesting that H. pylori infection reduces expression of the BabA receptor. The percentage of epithelium with bound bacteria was determined as described in Materials and Methods and is shown in the upper right of each panel.
Similar articles
- Dynamic Expression of the BabA Adhesin and Its BabB Paralog during Helicobacter pylori Infection in Rhesus Macaques.
Hansen LM, Gideonsson P, Canfield DR, Borén T, Solnick JV. Hansen LM, et al. Infect Immun. 2017 May 23;85(6):e00094-17. doi: 10.1128/IAI.00094-17. Print 2017 Jun. Infect Immun. 2017. PMID: 28396320 Free PMC article. - Dynamics of Lewis b binding and sequence variation of the babA adhesin gene during chronic Helicobacter pylori infection in humans.
Nell S, Kennemann L, Schwarz S, Josenhans C, Suerbaum S. Nell S, et al. mBio. 2014 Dec 16;5(6):e02281-14. doi: 10.1128/mBio.02281-14. mBio. 2014. PMID: 25516619 Free PMC article. - Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques.
Solnick JV, Hansen LM, Salama NR, Boonjakuakul JK, Syvanen M. Solnick JV, et al. Proc Natl Acad Sci U S A. 2004 Feb 17;101(7):2106-11. doi: 10.1073/pnas.0308573100. Epub 2004 Feb 3. Proc Natl Acad Sci U S A. 2004. PMID: 14762173 Free PMC article. - Adherence properties of Helicobacter pylori: impact on pathogenesis and adaptation to the host.
Odenbreit S. Odenbreit S. Int J Med Microbiol. 2005 Sep;295(5):317-24. doi: 10.1016/j.ijmm.2005.06.003. Int J Med Microbiol. 2005. PMID: 16173498 Review. - Helicobacter pylori BabA in adaptation for gastric colonization.
Ansari S, Yamaoka Y. Ansari S, et al. World J Gastroenterol. 2017 Jun 21;23(23):4158-4169. doi: 10.3748/wjg.v23.i23.4158. World J Gastroenterol. 2017. PMID: 28694656 Free PMC article. Review.
Cited by
- Role of mucus layers in gut infection and inflammation.
Hansson GC. Hansson GC. Curr Opin Microbiol. 2012 Feb;15(1):57-62. doi: 10.1016/j.mib.2011.11.002. Epub 2011 Dec 14. Curr Opin Microbiol. 2012. PMID: 22177113 Free PMC article. Review. - The Helicobacter pylori autotransporter ImaA (HP0289) modulates the immune response and contributes to host colonization.
Sause WE, Castillo AR, Ottemann KM. Sause WE, et al. Infect Immun. 2012 Jul;80(7):2286-96. doi: 10.1128/IAI.00312-12. Epub 2012 May 7. Infect Immun. 2012. PMID: 22566509 Free PMC article. - Recombination and DNA repair in Helicobacter pylori.
Dorer MS, Sessler TH, Salama NR. Dorer MS, et al. Annu Rev Microbiol. 2011;65:329-48. doi: 10.1146/annurev-micro-090110-102931. Annu Rev Microbiol. 2011. PMID: 21682641 Free PMC article. Review. - Analysis of a single Helicobacter pylori strain over a 10-year period in a primate model.
Liu H, Fero JB, Mendez M, Carpenter BM, Servetas SL, Rahman A, Goldman MD, Boren T, Salama NR, Merrell DS, Dubois A. Liu H, et al. Int J Med Microbiol. 2015 May;305(3):392-403. doi: 10.1016/j.ijmm.2015.03.002. Epub 2015 Mar 6. Int J Med Microbiol. 2015. PMID: 25804332 Free PMC article. - Divergent mechanisms of interaction of Helicobacter pylori and Campylobacter jejuni with mucus and mucins.
Naughton JA, Mariño K, Dolan B, Reid C, Gough R, Gallagher ME, Kilcoyne M, Gerlach JQ, Joshi L, Rudd P, Carrington S, Bourke B, Clyne M. Naughton JA, et al. Infect Immun. 2013 Aug;81(8):2838-50. doi: 10.1128/IAI.00415-13. Epub 2013 May 28. Infect Immun. 2013. PMID: 23716616 Free PMC article.
References
- Alm, R. A., L. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. - PubMed
- Aspholm-Hurtig, M., G. Dailide, M. Lahmann, A. Kalia, D. Ilver, N. Roche, S. Vikstrom, R. Sjostrom, S. Linden, A. Backstrom, C. Lundberg, A. Arnqvist, J. Mahdavi, U. J. Nilsson, B. Velapatino, R. H. Gilman, M. Gerhard, T. Alarcon, M. Lopez-Brea, T. Nakazawa, J. G. Fox, P. Correa, M. G. Dominguez-Bello, G. I. Perez-Perez, M. J. Blaser, S. Normark, I. Carlstedt, S. Oscarson, S. Teneberg, D. E. Berg, and T. Boren. 2004. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305:519-522. - PubMed
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1990. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI070803/AI/NIAID NIH HHS/United States
- T32 AI60555/AI/NIAID NIH HHS/United States
- T32 AI060555/AI/NIAID NIH HHS/United States
- R29 CA077955/CA/NCI NIH HHS/United States
- R01 DK063041/DK/NIDDK NIH HHS/United States
- P01 CA116087/CA/NCI NIH HHS/United States
- CA116087/CA/NCI NIH HHS/United States
- AI42081/AI/NIAID NIH HHS/United States
- R01 DK058587/DK/NIDDK NIH HHS/United States
- DK58587/DK/NIDDK NIH HHS/United States
- R01 AI042081/AI/NIAID NIH HHS/United States
- R01 CA077955/CA/NCI NIH HHS/United States
- DK63041/DK/NIDDK NIH HHS/United States
- CA77955/CA/NCI NIH HHS/United States
- AI070803/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical