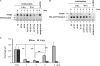Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase - PubMed (original) (raw)
Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase
You-Tong Wu et al. J Biol Chem. 2010.
Abstract
A group of phosphoinositide 3-kinase (PI3K) inhibitors, such as 3-methyladenine (3-MA) and wortmannin, have been widely used as autophagy inhibitors based on their inhibitory effect on class III PI3K activity, which is known to be essential for induction of autophagy. In this study, we systematically examined and compared the effects of these two inhibitors on autophagy under both nutrient-rich and deprivation conditions. To our surprise, 3-MA is found to promote autophagy flux when treated under nutrient-rich conditions with a prolonged period of treatment, whereas it is still capable of suppressing starvation-induced autophagy. We first observed that there are marked increases of the autophagic markers in cells treated with 3-MA in full medium for a prolonged period of time (up to 9 h). Second, we provide convincing evidence that the increase of autophagic markers is the result of enhanced autophagic flux, not due to suppression of maturation of autophagosomes or lysosomal function. More importantly, we found that the autophagy promotion activity of 3-MA is due to its differential temporal effects on class I and class III PI3K; 3-MA blocks class I PI3K persistently, whereas its suppressive effect on class III PI3K is transient. Because 3-MA has been widely used as an autophagy inhibitor in the literature, understanding the dual role of 3-MA in autophagy thus suggests that caution should be exercised in the application of 3-MA in autophagy study.
Figures
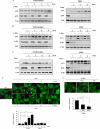
FIGURE 1.
Prolonged treatment with 3-MA in full medium leads to accumulation of autophagic markers. A, induction of LC3 I to II conversion by 3-MA in WT MEFs. WT MEFs were treated with 3-MA (5 m
m
) in full medium (left) or in EBSS (right; in the presence of 20 μ
m
CQ) for the indicated periods of time. Cell lysate was collected and subject to immunoblotting. B, similar treatments were also performed in L929 cells. C, effect of wortmannin (Wort) on LC3 I to II conversion. WT MEFs were treated with wortmannin (50 n
m
) either in full medium (left) or in EBSS (right; in the presence of 20 μ
m
CQ) for the indicated periods of time. D, induction of GFP-LC3 punctuation/aggregation in cells cultured in full medium by 3-MA but not by wortmannin. MEFs with stable expression of GFP-LC3 were treated with 3-MA (5 m
m
) or wortmannin (50 n
m
) as indicated and examined under a confocal microscope (×600, top). The enlarged area demonstrates the punctuated distribution of GFP-LC3 with a higher magnification. The number of GFP-LC3 puncta/cell were counted and presented (bottom; **, p < 0.01). E, effect of 3-MA and wortmannin on starvation-induced GFP-LC3 punctuation/aggregation. MEF cells with stable expression of GFP-LC3 as described in E were treated with 3-MA (5 m
m
) or wortmannin (50 n
m
) for 3 h under EBSS. The GFP-LC3 punctuation/aggregation was observed under a confocal microscope (×600, top). The GFP-LC3 puncta/cell were counted and presented (bottom; *, p < 0.05; **, p < 0.01).
FIGURE 2.
3-MA increases autophagic flux. A, 3-MA-induced LC3 I to II conversion is ATG5-dependent. Both WT and Atg5−/− MEF cells were incubated with 3-MA (5 m
m
) for up to 9 h in full medium. Cell lysates were subject to immunoblotting. B, Tet-off Atg5 MEFs with stable expression of GFP-LC3 were pretreated with or without doxycyclin (DOX) for 4 days, and then cells were treated with 3-MA (5 m
m
) for 9 h in full medium, and cell lysate was subject to Western blotting. C, Tet-off Atg5 MEFs were prepared as described in B and treated with 3-MA (5 m
m
) for 9 h. Cells were examined with a confocal microscope for GFP-LC3 punctuation/aggregation (top). The GFP-LC3 puncta/cell were counted and presented (bottom; **, p < 0.01). D, L929 cells stably transfected with tfLC3 (tfLC3-L929) were transfected with Atg7 siRNA for 48 h. Cells were then treated with 3-MA (5 m
m
) for 9 h in full medium. Cell lysates were subject to immunoblotting. E, tfLC3-L929 as described in D were treated with 3-MA (5 m
m
) for 9 h in full medium, and the GFP-LC3 punctuation/aggregation was observed under a confocal microscope (×600, top). The GFP-LC3 puncta/cell were counted and presented (bottom; **, p < 0.01). F, 3-MA promotes long lived protein degradation in cells cultured in full medium. HeLa cells were radiolabeled for 24 h with 0.05 mCi/ml
l
-[U-14C]valine. At the end of the labeling period, cells were rinsed three times with PBS. Cells were then incubated in full medium or in EBSS with 10 m
m
valine in the presence or absence of 10 m
m
3-MA for 4 h (top) or 8 h (bottom). The data in F are presented as means ± S.D. from three independent experiments and analyzed using Student's t test (*, p < 0.05; **, p < 0.01). Ctrl, control.
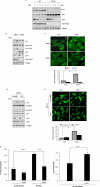
FIGURE 3.
3-MA does not affect lysosomal functions. A, effect of 3-MA on autolysosome maturation and lysosomal degradation. The L929 cells with stable expression of the tfLC3 construct (tfLC3-L929) were treated with CQ (20 μ
m
), 3-MA (5 m
m
), 3-MA + CQ and rapamycin (10 n
m
), or rapamycin + CQ for 9 h in full medium. The cells were examined using a confocal microscope (×600, top). The RFP- and GFP-LC3 puncta/cell were counted and presented (bottom; **, p < 0.01). B, effect of CQ on GFP-LC3 punctuation/aggregation induced by 3-MA. WT MEFs with stable expression of GFP-LC3 (same as in Fig. 2_B_ without DOX treatment) were treated with 3-MA (5 m
m
), CQ (20 μ
m
), or 3-MA + CQ for 9 h in full medium. The cells were examined by confocal microscopy (×600, left). The GFP-LC3 puncta/cell were counted and presented (right; **, p < 0.01). C, MEFs were treated as described in B, and the cell lysate were subjected to immunoblotting. D, effect of 3-MA on intralysosomal pH. WT MEF cells were treated with 3-MA (5 m
m
) or CQ (20 μ
m
) for 9 h in full medium, and the intralysosomal pH values were determined using a Lysosensor probe coupled with flow cytometry, as described under “Experimental Procedures.” E, effect of 3-MA on cathepsin B/L activity. WT MEFs were treated with 3-MA (5 m
m
), CQ (20 μ
m
), or cathepsin inhibitors (E64d + pepstatin A, 20 μg/ml each) for 9 h, and the lysate was subjected to the cathepsin B/L activity assay, as described under “Experimental Procedures.” The data in E are presented as means ± S.D. from three independent experiments and analyzed using Student's t test (**, p < 0.01). Ctrl, control.
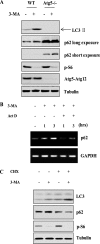
FIGURE 4.
3-MA up-regulates p62/SQSTM1 gene transcription. A, effect of 3-MA on p62 protein level in Atg5−/− MEFs. WT and Atg5−/− MEFs were treated with 3-MA (5 m
m
) for 9 h in full medium, and then cell lysate was collected and subjected to immunoblotting. B, effect of 3-MA on p62 mRNA level. WT MEFs were treated with 3-MA (5 m
m
), actinomycin D (Act D) (10 μ
m
), or 3-MA + ActD for 1 or 3 h in full medium. The p62 mRNA level was determined by reverse transcription-PCR. C, CHX blocks 3-MA-induced increase of p62 protein level. WT MEFs were treated with 3-MA (5 m
m
), CHX (10 μ
m
), or 3-MA + CHX for 9 h in full medium, and cell lysate was subjected to immunoblotting. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
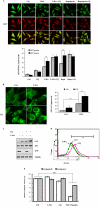
FIGURE 5.
3-MA inhibits class I and class III PI3K in different temporal patterns. A, the effect of 3-MA and wortmannin (Wort) on production of PI3P. WT MEFs were treated in EBSS or with 3-MA (5 m
m
), wortmannin (50 n
m
) in full medium as indicated. The levels of PI3P were measured using the PI3P mass strip kit as described under “Experimental Procedures.” B, the effect of 3-MA and wortmannin on production of PI(3,4,5)P3. WT MEFs were treated as in A, and the levels of PI(3,4,5)P3 were measured using the PI(3,4,5)P3 mass strip kit. C, WT MEFs were treated with 3-MA (5 m
m
) or wortmannin (50 m
m
) for 1, 3, 6, and 9 h in full medium, and the intracellular level of PI(3,4,5)P3 was measured using a chromatography-based method, as described under “Experimental Procedures.” The changes of PI(3,4,5)P3 are shown as relative peak height and are presented as means ± S.D. from three independent experiments and analyzed using Student's t test (**, p < 0.01).
FIGURE 6.
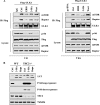
3-MA disrupts the function of mTOR complex I. A, 3-MA suppresses the interaction between ULK1 and mTOR complex 1. HEK293T cells were transfected with FLAG-ULK1 for 36 h, and cells were then treated in EBSS or with 3-MA (5 m
m
), wortmannin (50 n
m
) in full medium for 1 h (left) or 9 h (right). The cell lysate was subjected to immunoprecipitation (IP) using an antibody against FLAG, followed by immunoblotting. A fraction of the cell lysate was used for immunoblotting as indicated. B, the effect of 3-MA on autophagy in _Tsc2_−/− MEFs. WT and _Tsc2_−/− MEFs were treated with 3-MA (5 m
m
) or rapamycin (10 n
m
) for 9 h, and the cell lysate was subject to immunoblotting.
Similar articles
- The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes.
Heckmann BL, Yang X, Zhang X, Liu J. Heckmann BL, et al. Br J Pharmacol. 2013 Jan;168(1):163-71. doi: 10.1111/j.1476-5381.2012.02110.x. Br J Pharmacol. 2013. PMID: 22817685 Free PMC article. - Inhibitors of phosphatidylinositol 3'-kinases promote mitotic cell death in HeLa cells.
Hou H, Zhang Y, Huang Y, Yi Q, Lv L, Zhang T, Chen D, Hao Q, Shi Q. Hou H, et al. PLoS One. 2012;7(4):e35665. doi: 10.1371/journal.pone.0035665. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545128 Free PMC article. - Cocaine-mediated microglial activation involves the ER stress-autophagy axis.
Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S. Guo ML, et al. Autophagy. 2015;11(7):995-1009. doi: 10.1080/15548627.2015.1052205. Autophagy. 2015. PMID: 26043790 Free PMC article. - Inhibition of autophagy in mitotic animal cells.
Eskelinen EL, Prescott AR, Cooper J, Brachmann SM, Wang L, Tang X, Backer JM, Lucocq JM. Eskelinen EL, et al. Traffic. 2002 Dec;3(12):878-93. doi: 10.1034/j.1600-0854.2002.31204.x. Traffic. 2002. PMID: 12453151
Cited by
- Pharmaceutical Agents for Targeting Autophagy and Their Applications in Clinics.
Kench U, Sologova S, Smolyarchuk E, Prassolov V, Spirin P. Kench U, et al. Pharmaceuticals (Basel). 2024 Oct 11;17(10):1355. doi: 10.3390/ph17101355. Pharmaceuticals (Basel). 2024. PMID: 39458996 Free PMC article. Review. - Pyrroloquinoline Quinone Inhibits Rotenone-Induced Microglia Inflammation by Enhancing Autophagy.
Zhang Q, Zhou J, Shen M, Xu H, Yu S, Cheng Q, Ding F. Zhang Q, et al. Molecules. 2020 Sep 23;25(19):4359. doi: 10.3390/molecules25194359. Molecules. 2020. PMID: 32977419 Free PMC article. - Ferritin Is Secreted from Primary Cultured Astrocyte in Response to Iron Treatment via TRPML1-Mediated Exocytosis.
Yu X, Xiao Z, Xie J, Xu H. Yu X, et al. Cells. 2023 Oct 25;12(21):2519. doi: 10.3390/cells12212519. Cells. 2023. PMID: 37947597 Free PMC article. - Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension.
Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, Konduri GG. Teng RJ, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Apr 1;302(7):L651-63. doi: 10.1152/ajplung.00177.2011. Epub 2012 Jan 13. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22245997 Free PMC article. - Autophagy activation is involved in 3,4-methylenedioxymethamphetamine ('ecstasy')--induced neurotoxicity in cultured cortical neurons.
Li IH, Ma KH, Weng SJ, Huang SS, Liang CM, Huang YS. Li IH, et al. PLoS One. 2014 Dec 31;9(12):e116565. doi: 10.1371/journal.pone.0116565. eCollection 2014. PLoS One. 2014. PMID: 25551657 Free PMC article.
References
- Mizushima N. (2007) Genes Dev. 21, 2861–2873 - PubMed
- Xie Z., Klionsky D. J. (2007) Nat. Cell Biol. 9, 1102–1109 - PubMed
- Pattingre S., Espert L., Biard-Piechaczyk M., Codogno P. (2008) Biochimie 90, 313–323 - PubMed
- Inoki K., Guan K. L. (2006) Trends Cell Biol. 16, 206–212 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources