Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients - PubMed (original) (raw)
Multicenter Study
. 2010 Mar 10;28(8):1287-93.
doi: 10.1200/JCO.2009.25.7246. Epub 2010 Feb 1.
Taisei Mushiroda, Chiyo K Imamura, Naoya Hosono, Tatsuhiko Tsunoda, Michiaki Kubo, Yusuke Tanigawara, David A Flockhart, Zeruesenay Desta, Todd C Skaar, Fuminori Aki, Koichi Hirata, Yuichi Takatsuka, Minoru Okazaki, Shozo Ohsumi, Takashi Yamakawa, Mitsunori Sasa, Yusuke Nakamura, Hitoshi Zembutsu
Affiliations
- PMID: 20124171
- PMCID: PMC4872305
- DOI: 10.1200/JCO.2009.25.7246
Multicenter Study
Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients
Kazuma Kiyotani et al. J Clin Oncol. 2010.
Abstract
Purpose: The clinical efficacy of tamoxifen is suspected to be influenced by the activity of drug-metabolizing enzymes and transporters involved in the formation, metabolism, and elimination of its active forms. We investigated relationships of polymorphisms in transporter genes and CYP2D6 to clinical outcome of patients receiving tamoxifen.
Patients and methods: We studied 282 patients with hormone receptor-positive, invasive breast cancer receiving tamoxifen monotherapy, including 67 patients who have been previously reported. We investigated the effects of allelic variants of CYP2D6 and haplotype-tagging single nucleotide polymorphisms (tag-SNPs) of ABCB1, ABCC2, and ABCG2 on recurrence-free survival using the Kaplan-Meier method and Cox regression analysis. Plasma concentrations of tamoxifen metabolites were measured in 98 patients receiving tamoxifen 20 mg/d.
Results: CYP2D6 variants were significantly associated with shorter recurrence-free survival (P = .000036; hazard ratio [HR] = 9.52; 95% CI, 2.79 to 32.45 in patients with two variant alleles v patients without variant alleles). Among 51 tag-SNPs in transporter genes, a significant association was found at rs3740065 in ABCC2 (P = .00017; HR = 10.64; 95% CI, 1.44 to 78.88 in patients with AA v GG genotypes). The number of risk alleles of CYP2D6 and ABCC2 showed cumulative effects on recurrence-free survival (P = .000000055). Patients carrying four risk alleles had 45.25-fold higher risk compared with patients with <or= one risk allele. CYP2D6 variants were associated with lower plasma levels of endoxifen and 4-hydroxytamoxifen (P = .0000043 and .00052), whereas no significant difference was found among ABCC2 genotype groups.
Conclusion: Our results suggest that polymorphisms in CYP2D6 and ABCC2 are important predictors for the prognosis of patients with breast cancer treated with tamoxifen.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
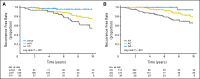
Fig 1.
Kaplan-Meier estimates of recurrence-free survival for CYP2D6 and ABCC2 rs3740065 genotypes. (A) Comparison of 282 patients with wt/wt, wt/V, and V/V of CYP2D6. (B) Comparison of 282 patients with AA, AG, and GG genotypes at rs3740065 in ABCC2.
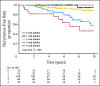
Fig 2.
Kaplan-Meier estimates of recurrence-free survival for combination effects of CYP2D6 and ABCC2 genotypes. The patients were classified into five groups (zero, one, two, three, or four alleles) based on the number of risk alleles of these two genes.
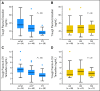
Fig 3.
Steady-state plasma concentrations of (A, B) endoxifen and (C, D) 4-hydroxytamoxifen (4-OH tamoxifen) according to genotypes. (A, C) Comparison among wt/wt, wt/V, and V/V of CYP2D6. (B, D) Comparison among AA, AG, and GG genotypes at rs3740065 in ABCC2. The horizontal line indicates the median concentration, the box covers the 25th to 75th percentiles, and the maximum length of each whisker is 1.5 × the interquartile range; dots outside the whiskers are outliers.
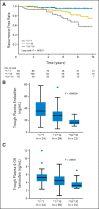
Fig A1.
Association of CYP2D6*10 with recurrence-free survival and plasma concentrations of tamoxifen metabolites in patients with breast cancer. (A) Kaplan-Meier estimates of recurrence-free survival for patients with CYP2D6*1/*1, *1/*10, and *10/*10 genotypes. Steady-state plasma concentrations of (B) endoxifen and (C) 4-hydroxytamoxifen (4-OH tamoxifen) according to CYP2D6*10 genotypes. The horizontal line indicates the median concentration, the box covers the 25th to 75th percentiles, and the maximum length of each whisker is 1.5 × the interquartile range; dots outside the whiskers are outliers.
Comment in
- Evidence and practice regarding the role for CYP2D6 inhibition in decisions about tamoxifen therapy.
Lash TL, Rosenberg CL. Lash TL, et al. J Clin Oncol. 2010 Mar 10;28(8):1273-5. doi: 10.1200/JCO.2009.26.7906. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124162 No abstract available. - ABCC2 and clinical outcome of tamoxifen therapy.
Lang T, Schroth W, Brauch H, Schwab M. Lang T, et al. J Clin Oncol. 2010 Sep 1;28(25):e448; author reply e449. doi: 10.1200/JCO.2010.29.6665. Epub 2010 Jul 12. J Clin Oncol. 2010. PMID: 20625134 No abstract available.
Similar articles
- Lack of any association between functionally significant CYP2D6 polymorphisms and clinical outcomes in early breast cancer patients receiving adjuvant tamoxifen treatment.
Park IH, Ro J, Park S, Lim HS, Lee KS, Kang HS, Jung SY, Lee S. Park IH, et al. Breast Cancer Res Treat. 2012 Jan;131(2):455-61. doi: 10.1007/s10549-011-1425-2. Epub 2011 Mar 25. Breast Cancer Res Treat. 2012. PMID: 21437611 - Genetic polymorphisms of CYP2D6 increase the risk for recurrence of breast cancer in patients receiving tamoxifen as an adjuvant therapy.
Damodaran SE, Pradhan SC, Umamaheswaran G, Kadambari D, Reddy KS, Adithan C. Damodaran SE, et al. Cancer Chemother Pharmacol. 2012 Jul;70(1):75-81. doi: 10.1007/s00280-012-1891-1. Epub 2012 May 24. Cancer Chemother Pharmacol. 2012. PMID: 22623212 - The risk of recurrence in breast cancer patients treated with tamoxifen: polymorphisms of CYP2D6 and ABCB1.
Teh LK, Mohamed NI, Salleh MZ, Rohaizak M, Shahrun NS, Saladina JJ, Shia JK, Roslan H, Sood S, Rajoo TS, Muniandy SP, Henry G, Ngow HA, Hla U KT, Din J. Teh LK, et al. AAPS J. 2012 Mar;14(1):52-9. doi: 10.1208/s12248-011-9313-6. Epub 2011 Dec 20. AAPS J. 2012. PMID: 22183189 Free PMC article. - [Advances in the research of pharmacogenomics of tamoxifen].
Xiong W, Zhao JJ, Wang L, Jiang XH, Tao XQ. Xiong W, et al. Yao Xue Xue Bao. 2016 Sep;51(9):1356-67. Yao Xue Xue Bao. 2016. PMID: 29924509 Review. Chinese. - CYP2D6 genotype and tamoxifen response for breast cancer: a systematic review and meta-analysis.
Lum DW, Perel P, Hingorani AD, Holmes MV. Lum DW, et al. PLoS One. 2013 Oct 2;8(10):e76648. doi: 10.1371/journal.pone.0076648. eCollection 2013. PLoS One. 2013. PMID: 24098545 Free PMC article. Review.
Cited by
- Enriching Medication Review with a Pharmacogenetic Profile - A Case of Tamoxifen Adverse Drug Reactions.
Jeiziner C, Stäuble CK, Lampert ML, Hersberger KE, Meyer Zu Schwabedissen HE. Jeiziner C, et al. Pharmgenomics Pers Med. 2021 Feb 19;14:279-286. doi: 10.2147/PGPM.S285807. eCollection 2021. Pharmgenomics Pers Med. 2021. PMID: 33642872 Free PMC article. - Application of PBPK Modeling and Virtual Clinical Study Approaches to Predict the Outcomes of CYP2D6 Genotype-Guided Dosing of Tamoxifen.
Nakamura T, Toshimoto K, Lee W, Imamura CK, Tanigawara Y, Sugiyama Y. Nakamura T, et al. CPT Pharmacometrics Syst Pharmacol. 2018 Jul;7(7):474-482. doi: 10.1002/psp4.12307. Epub 2018 Jun 19. CPT Pharmacometrics Syst Pharmacol. 2018. PMID: 29920987 Free PMC article. - Impact of systemic adjuvant therapy and CYP2D6 activity on mammographic density in a cohort of tamoxifen-treated breast cancer patients.
Thorén L, Eriksson M, Lindh JD, Czene K, Bergh J, Eliasson E, Hall P, Margolin S. Thorén L, et al. Breast Cancer Res Treat. 2021 Dec;190(3):451-462. doi: 10.1007/s10549-021-06386-2. Epub 2021 Sep 27. Breast Cancer Res Treat. 2021. PMID: 34570302 Free PMC article. - Comprehensive assessment of cytochromes P450 and transporter genetics with endoxifen concentration during tamoxifen treatment.
Marcath LA, Deal AM, Van Wieren E, Danko W, Walko CM, Ibrahim JG, Weck KE, Jones DR, Desta Z, McLeod HL, Carey LA, Irvin WJ Jr, Hertz DL. Marcath LA, et al. Pharmacogenet Genomics. 2017 Nov;27(11):402-409. doi: 10.1097/FPC.0000000000000311. Pharmacogenet Genomics. 2017. PMID: 28877533 Free PMC article. - CYP2D6 gene variants: association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen.
Abraham JE, Maranian MJ, Driver KE, Platte R, Kalmyrzaev B, Baynes C, Luccarini C, Shah M, Ingle S, Greenberg D, Earl HM, Dunning AM, Pharoah PD, Caldas C. Abraham JE, et al. Breast Cancer Res. 2010;12(4):R64. doi: 10.1186/bcr2629. Epub 2010 Aug 23. Breast Cancer Res. 2010. PMID: 20731819 Free PMC article.
References
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. - PubMed
- Borgna JL, Rochefort H. Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem. 1981;256:859–868. - PubMed
- Lien EA, Solheim E, Lea OA, et al. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49:2175–2183. - PubMed
- Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases