Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats - PubMed (original) (raw)
Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats
Zhongwu Liu et al. J Cereb Blood Flow Metab. 2010 Jul.
Abstract
We investigated axonal plasticity in the bilateral motor cortices in rats after unilateral stroke and bone marrow stromal cell (BMSC) treatment. Rats were subjected to permanent right middle cerebral artery occlusion followed by intravenous administration of phosphate-buffered saline or BMSCs 1 day later. Adhesive-removal test and modified neurologic severity score were performed weekly to monitor limb functional deficit and recovery. Anterograde tracing with biotinylated dextran amine injected into the right motor cortex was used to assess axonal sprouting in the contralateral motor cortex and ipsilateral rostral forelimb area. Animals were killed 28 days after stroke. Progressive functional recovery was significantly enhanced by BMSCs. Compared with normal animals, axonal density in both contralateral motor cortex and ipsilateral rostral forelimb area significantly increased after stroke. Bone marrow stromal cells markedly enhanced such interhemispheric and intracortical connections. However, labeled transcallosal axons in the corpus callosum were not altered with either stroke or treatment. Both interhemispheric and intracortical axonal sprouting were significantly and highly correlated with behavioral outcome after stroke. This study suggests that, after stroke, cortical neurons surviving in the peri-infarct motor cortex undergo axonal sprouting to restore connections between different cerebral areas. Bone marrow stromal cells enhance axonal plasticity, which may underlie neurologic functional improvement.
Figures
Figure 1
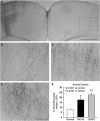
Permanent MCAo induced ischemic lesion in the rat brain. (A) A representative rat coronal brain section shows the ischemic lesion 4 weeks after MCAo. An arrow indicates the location of BDA injection in the right cortex. Rectangle fields in the left cortex (1) and corpus callosum (2) indicate the position of the photomicrograph appearing in Figures 2 and 3, respectively. (B) Quantitative data show no statistical difference in the ischemic lesion size between animal groups treated with PBS or BMSCs. Scale bar=2 mm.
Figure 2
Transcallosal axons in the contralateral cortex labeled with BDA intracortical injection. (A) A representative image shows the BDA-positive labeling in a normal rat brain. The BDA solution was injected into the right cortex 7 days before the kill. The BDA-labeled axons in the boxed area in the contralateral CFA were measured as proportional areas, as shown in B for normal, in C for MCAo, and in D for BMSC-treated groups. The axonal density was significantly increased 28 days after MCAo compared with normal animals (C and E). Bone marrow stromal cell administration further enhanced such stroke-induced axonal reorganization (D and E). Scale bar=1 mm in A and 250 _μ_m in B–D.
Figure 3
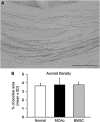
Axonal labeling in the corpus callosum. (A) A representative image shows the transcallosal axons in the middle portion of corpus callosum enlarged from the box 2 in Figure 1A. (B) There are no significant differences in axonal quantification among normal animals and ischemic rats with or without BMSC treatment. Scale bar=150 _μ_m.
Figure 4
Coronal sections showing intracortical axonal connections in the right RFA originating from the ipsilateral CFA. In normal rats, few axons were detected in the cortex at the RFA level with BDA injection into the cortex of the CFA (A). Biotinylated dextran amine-positive axons were increased in the ischemic animals treated with PBS (B) and BMSCs (C). Quantitative data showed a significant enhancing effect on both MCAo lesion and BMSC treatment, respectively (D). Insets in A, schematic drawing of the rat brain dorsal view showing the RFA region from which slices were obtained (lines) and BDA-injection position in the CFA (dot). Cross-hatching indicates the ischemic lesion. Scale bar=250 _μ_m.
Similar articles
- Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment.
Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Liu Z, et al. Stroke. 2008 Sep;39(9):2571-7. doi: 10.1161/STROKEAHA.107.511659. Epub 2008 Jul 10. Stroke. 2008. PMID: 18617661 Free PMC article. - Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke.
Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Shen LH, et al. Neuroscience. 2006;137(2):393-9. doi: 10.1016/j.neuroscience.2005.08.092. Epub 2005 Nov 17. Neuroscience. 2006. PMID: 16298076 - Molecular, cellular and functional events in axonal sprouting after stroke.
Carmichael ST, Kathirvelu B, Schweppe CA, Nie EH. Carmichael ST, et al. Exp Neurol. 2017 Jan;287(Pt 3):384-394. doi: 10.1016/j.expneurol.2016.02.007. Epub 2016 Feb 10. Exp Neurol. 2017. PMID: 26874223 Free PMC article. Review. - Plasticity of cortical projections after stroke.
Carmichael ST. Carmichael ST. Neuroscientist. 2003 Feb;9(1):64-75. doi: 10.1177/1073858402239592. Neuroscientist. 2003. PMID: 12580341 Review.
Cited by
- The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice.
Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, Chopp M. Ding X, et al. J Cereb Blood Flow Metab. 2013 Jul;33(7):1015-24. doi: 10.1038/jcbfm.2013.50. Epub 2013 Apr 3. J Cereb Blood Flow Metab. 2013. PMID: 23549381 Free PMC article. - Correlating interleukin-10 promoter gene polymorphisms with human cerebral infarction onset.
Jiang XH, Lin KX, Zhang YX, Chen RH, Liu N. Jiang XH, et al. Neural Regen Res. 2015 Nov;10(11):1809-13. doi: 10.4103/1673-5374.170308. Neural Regen Res. 2015. PMID: 26807116 Free PMC article. - Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke.
Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, Liu XS, Zhang Y, Roberts C, Zhang ZG. Ueno Y, et al. Stroke. 2012 Aug;43(8):2221-8. doi: 10.1161/STROKEAHA.111.646224. Epub 2012 May 22. Stroke. 2012. PMID: 22618383 Free PMC article. - Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke.
Hermann DM, Xin W, Bähr M, Giebel B, Doeppner TR. Hermann DM, et al. Theranostics. 2022 Jul 18;12(13):5776-5802. doi: 10.7150/thno.73931. eCollection 2022. Theranostics. 2022. PMID: 35966580 Free PMC article. Review. - Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury.
Ning R, Xiong Y, Mahmood A, Zhang Y, Meng Y, Qu C, Chopp M. Ning R, et al. Brain Res. 2011 Apr 12;1384:140-50. doi: 10.1016/j.brainres.2011.01.099. Epub 2011 Feb 3. Brain Res. 2011. PMID: 21295557 Free PMC article.
References
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. - PubMed
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. - PubMed
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. - PubMed
Publication types
MeSH terms
Grants and funding
- P01 NS042345/NS/NINDS NIH HHS/United States
- P01 NS042345-05/NS/NINDS NIH HHS/United States
- R01 NS066041/NS/NINDS NIH HHS/United States
- P01 NS42345/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources