Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration - PubMed (original) (raw)
Review
Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration
Ronald W Matheny Jr et al. Endocrinology. 2010 Mar.
Abstract
The discovery that IGF-I mRNAs encoding isoforms of the pro-IGF-I molecule are differentially regulated in response to mechanical stress in skeletal muscle has been the impetus for a number of studies designed to demonstrate that alternative splicing of IGF-I pre-mRNA involving exons 4, 5, and 6 gives rise to a unique peptide derived from pro-IGF-I that plays a novel role in myoblast proliferation. Research suggests that after injury to skeletal muscle, the IGF-IEb mRNA splice variant is up-regulated initially, followed by up-regulation of the IGF-IEa splice variant at later time points. Up-regulation of IGF-IEb mRNA correlates with markers of satellite cell and myoblast proliferation, whereas up-regulation of IGF-IEa mRNA is correlated with differentiation to mature myofibers. Due to the apparent role of IGF-IEb up-regulation in muscle remodeling, IGF-IEb mRNA was also named mechano-growth factor (MGF). A synthetically manufactured peptide (also termed MGF) corresponding to the 24 most C-terminal residues of IGF-IEb has been shown to promote cellular proliferation and survival. However, no analogous peptide product of the Igf1 gene has been identified in or isolated from cultured cells, their conditioned medium, or in vivo animal tissues or biological fluids. This review will discuss the relationship of the Igf1 gene to MGF and will differentiate actions of synthetic MGF from any known product of Igf1. Additionally, the role of MGF in satellite cell activation, aging, neuroprotection, and signaling will be discussed. A survey of outstanding questions relating to MGF will also be provided.
Figures
Figure 1
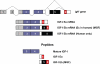
Splicing and peptide products of the Igf1 gene. The Igf1 gene contains six exons; exons 1 and 2 (gray) serve as alternative leader exons at the 5′-end of the mRNA, whereas exons 3 and 4 (purple) are common to all splice variants. Several transcripts can be derived by alternative splicing at the C terminus, including exon 4 spliced directly to exon 6 (Ea) or exon 4 spliced to exon 5 spliced to exon 6. IGF-I mRNA containing exon 5 is referred to as Eb in rodents (Ec in humans) and has also been referred to as MGF (33). In humans, but not rodents, mRNAs containing exon 5 spliced to exon 4 have been identified (9) and are designated as Eb. Peptide products, derived from pro-IGF-I and referred to in the text, are shown.
Figure 2
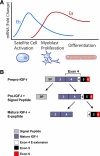
The MGF hypothesis. A, The MGF hypothesis suggests that after muscle injury such as that caused by exercise, the Igf1 gene is first spliced toward the Eb (MGF) splice variant. Presumably, IGF-IEb mRNA will then be translated and processed to yield mature IGF-I as well as the autonomous C-terminal MGF peptide. The MGF peptide is then responsible for activating quiescent satellite cells to enter the cell cycle and develop into mononucleated myoblasts; MGF then promotes myoblast proliferation. However, MGF also inhibits differentiation; thus, levels of MGF must decrease for differentiation to occur. During the myoblast proliferative stage, splicing of the Igf1 gene is increasingly shifted toward the Ea splice variant, which promotes further myoblast proliferation and potentiates myoblast differentiation into multinucleated myotubes. Thus, the MGF hypothesis suggests that MGF is responsible for satellite cell activation and proliferation, whereas IGF-IEa is responsible for differentiation. The events outlined above describe the MGF hypothesis, but little has been shown with respect to endogenous proteins acting in the manner described. Thus, although the graph depicts cellular repair events that coincide with IGF-I splice variant mRNA levels, extreme caution should be employed when extrapolating mRNA data to actions at the protein level, as the MGF hypothesis does. In particular, it is essential to realize that levels of IGF-IEb mRNA are far lower than levels of IGF-IEa mRNA. Panel A does not reflect this, but rather illustrates the fold changes in the respective splice variants compared to unstimulated levels. B, Proposed generation of MGF. Processing of pre-pro-IGF-I contains at least two general steps; the first step involves removal of the signal peptide, and a second step liberates the E-peptide from mature IGF-I.
Figure 3
Required step for derivation of MGF from the rest of the E-peptide. For MGF to exist as an endogenous 25-amino-acid peptide (24 amino acids in humans due to absence of one codon within exon 5; human sequence is shown), a processing step must occur that removes the 16-amino-acid E-extension from the peptide generated from exon 5 and exon 6 (see also Table 2); this step has not been observed in any system to date. Additionally, there is no identified sequence or motif within the intact 41-amino-acid E-peptide that would suggest this step occurs. For an endogenous peptide equivalent to the synthetic MGF to exist, proteolytic cleavage must occur that liberates those residues encoded by exon 5–6 (blue text) from those encoded by the exon 4 E-extension (black text). No furin or furin-like convertase, which are known to process pro-IGF-I, cleave scissile bonds on the carboxyl end of lysine or on the amino end of tyrosine.
Figure 4
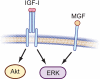
IGF-IR and MGF signaling are distinct. Signaling through the IGF-IR activates a number of intracellular signaling pathways including PI3K/Akt and MAPK kinase/ERK. Conversely, MGF synthetic peptide has been proposed to signal through an IGF-IR-independent mechanism to activate ERK, but not Akt (31,80). A specific MGF receptor has not yet been identified; thus, that monomeric MGF binds to a single-chain transmembrane receptor as illustrated, is speculative.
Similar articles
- [Expression of mechano-growth factor and its roles in tissue repairs and regeneration].
Zhang B, Song G, Luo Q, Yang L. Zhang B, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012 May;26(5):617-20. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012. PMID: 22702061 Review. Chinese. - Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage.
Hill M, Goldspink G. Hill M, et al. J Physiol. 2003 Jun 1;549(Pt 2):409-18. doi: 10.1113/jphysiol.2002.035832. Epub 2003 Apr 11. J Physiol. 2003. PMID: 12692175 Free PMC article. - Expression of IGF-1 isoforms after exercise-induced muscle damage in humans: characterization of the MGF E peptide actions in vitro.
Philippou A, Papageorgiou E, Bogdanis G, Halapas A, Sourla A, Maridaki M, Pissimissis N, Koutsilieris M. Philippou A, et al. In Vivo. 2009 Jul-Aug;23(4):567-75. In Vivo. 2009. PMID: 19567392 - Muscle mechano growth factor is preferentially induced by growth hormone in growth hormone-deficient lit/lit mice.
Iida K, Itoh E, Kim DS, del Rincon JP, Coschigano KT, Kopchick JJ, Thorner MO. Iida K, et al. J Physiol. 2004 Oct 15;560(Pt 2):341-9. doi: 10.1113/jphysiol.2004.069500. Epub 2004 Aug 12. J Physiol. 2004. PMID: 15308683 Free PMC article. - Insulin-like growth factor-I E peptides: implications for aging skeletal muscle.
Velloso CP, Harridge SD. Velloso CP, et al. Scand J Med Sci Sports. 2010 Feb;20(1):20-7. doi: 10.1111/j.1600-0838.2009.00997.x. Scand J Med Sci Sports. 2010. PMID: 19883387 Review.
Cited by
- β-Arrestin scaffolds and signaling elements essential for the obestatin/GPR39 system that determine the myogenic program in human myoblast cells.
Santos-Zas I, Gurriarán-Rodríguez U, Cid-Díaz T, Figueroa G, González-Sánchez J, Bouzo-Lorenzo M, Mosteiro CS, Señarís J, Casanueva FF, Casabiell X, Gallego R, Pazos Y, Mouly V, Camiña JP. Santos-Zas I, et al. Cell Mol Life Sci. 2016 Feb;73(3):617-35. doi: 10.1007/s00018-015-1994-z. Epub 2015 Jul 27. Cell Mol Life Sci. 2016. PMID: 26211463 Free PMC article. - Human Eb peptide: not just a by-product of pre-pro-IGF1b processing?
Durzyńska J, Wardziński A, Koczorowska M, Goździcka-Józefiak A, Barton ER. Durzyńska J, et al. Horm Metab Res. 2013 Jun;45(6):415-22. doi: 10.1055/s-0032-1331699. Epub 2013 Jan 18. Horm Metab Res. 2013. PMID: 23335048 Free PMC article. - Oncogenic Role of the Ec Peptide of the IGF-1Ec Isoform in Prostate Cancer.
Armakolas A, Kaparelou M, Dimakakos A, Papageorgiou E, Armakolas N, Antonopoulos A, Petraki C, Lekarakou M, Lelovas P, Stathaki M, Psarros C, Donta I, Galanos PS, Msaouel P, Gorgoulis VG, Koutsilieris M. Armakolas A, et al. Mol Med. 2015 Jan 6;21(1):167-79. doi: 10.2119/molmed.2014.00222. Mol Med. 2015. PMID: 25569803 Free PMC article. - Possible role of the Ec peptide of IGF‑1Ec in cartilage repair.
Armakolas N, Dimakakos A, Armakolas A, Antonopoulos A, Koutsilieris M. Armakolas N, et al. Mol Med Rep. 2016 Oct;14(4):3066-72. doi: 10.3892/mmr.2016.5627. Epub 2016 Aug 12. Mol Med Rep. 2016. PMID: 27571686 Free PMC article. - Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization.
Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, Rosenthal N. Tonkin J, et al. Mol Ther. 2015 Jul;23(7):1189-1200. doi: 10.1038/mt.2015.66. Epub 2015 Apr 21. Mol Ther. 2015. PMID: 25896247 Free PMC article.
References
- Kooijman R 2006 Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev 17:305–323 - PubMed
- Jones JI, Clemmons DR 1995 Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 - PubMed
- Adamo ML, Ben-Hur H, LeRoith D, Roberts Jr CT 1991 Transcription initiation in the two leader exons of the rat IGF-I gene occurs from disperse versus localized sites. Biochem Biophys Res Commun 176:887–893 - PubMed
- Rotwein P, Bichell DP, Kikuchi K 1993 Multifactorial regulation of IGF-I gene expression. Mol Reprod Dev 35:358–363; discussion 363–354 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous