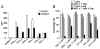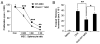Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression - PubMed (original) (raw)
Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression
Guangwen Ren et al. J Immunol. 2010.
Abstract
Cell-cell adhesion mediated by ICAM-1 and VCAM-1 is critical for T cell activation and leukocyte recruitment to the inflammation site and, therefore, plays an important role in evoking effective immune responses. However, we found that ICAM-1 and VCAM-1 were critical for mesenchymal stem cell (MSC)-mediated immunosuppression. When MSCs were cocultured with T cells in the presence of T cell Ag receptor activation, they significantly upregulated the adhesive capability of T cells due to the increased expression of ICAM-1 and VCAM-1. By comparing the immunosuppressive effect of MSCs toward various subtypes of T cells and the expression of these adhesion molecules, we found that the greater expression of ICAM-1 and VCAM-1 by MSCs, the greater the immunosuppressive capacity that they exhibited. Furthermore, ICAM-1 and VCAM-1 were found to be inducible by the concomitant presence of IFN-gamma and inflammatory cytokines (TNF-alpha or IL-1). Finally, MSC-mediated immunosuppression was significantly reversed in vitro and in vivo when the adhesion molecules were genetically deleted or functionally blocked, which corroborated the importance of cell-cell contact in immunosuppression by MSCs. Taken together, these findings reveal a novel function of adhesion molecules in immunoregulation by MSCs and provide new insights for the clinical studies of antiadhesion therapies in various immune disorders.
Conflict of interest statement
Disclosures: The authors have no financial conflicts of interest.
Figures
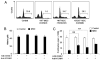
FIGURE 1
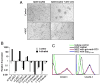
Expression of ICAM-1 and VCAM-1 in MSCs was greatly induced by T cell products. A, Fresh C57BL/6 splenocytes were stimulated with or without anti-CD3 (1 μg/ml) and cultured in the presence or absence of MSCs derived from C57BL/6 mice at a 20:1 ratio (splenocytes/MSCs). The cells were examined microscopically after 48 h (original magnification ×200). Representative of 10 independent experiments. B, Expression of adhesion molecules at mRNA levels in MSCs cocultured with activated splenocytes. MSCs were incubated for 48 h in the presence of fresh splenocytes activated by anti-CD3 (1 μg/ml). MSCs not exposed to activated splenocytes were used as controls. After removing the nonadhesive lymphocytes, the MSCs were purified by a CD45-microbeads kit using a negative selection-based MACS sorting. The gene expression of the adhesion molecules in purified MSCs (>95% pure based on flow cytometry test) and the control MSCs were analyzed by real-time PCR and compared with β-actin mRNA, defined as 1000 arbitrary unit. Representative of three independent experiments. C, Expression of ICAM-1 and VCAM-1 assayed by flow cytometry. MSCs were cultured with or without fresh splenocytes as in B or supplemented with SupCD3-act (50% of total volume) for 24 h. The expression of ICAM-1 and VCAM-1 in MSCs was detected by flow cytometry using electronic gating to exclude lymphocytes. Untreated MSCs served as a control. Data shown are mean ± SD of a representative of five experiments.
FIGURE 2
The effect of supernatants from different T cell subtypes on the expression of ICAM-1 and VCAM-1 in MSCs. A, MSCs were cultured with Suppan-T, SupCD4, SupCD8, SupTh1, SupTh2, or SupTh17 at a 1:1 dilution. ICAM-1/VCAM-1 expression in MSCs was measured by flow cytometry 24 h after treatment, and median fluorescence intensity (MFI) was obtained. Data shown are means ± SD of a representative of three experiments. B, To determine the effect of different T cell supernatants on MSC-mediated immunosuppression, after 8 h of treatment with the supernatants as in A, the respective T cell blasts were added at a 20:1 ratio to MSC cultures along with IL-2 (200 U/ml). Cell proliferation was assessed by [3H]-TdR incorporation after an additional 8 h. Values are means ± SD of five replicate wells from a representative of three experiments.
FIGURE 3
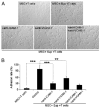
Increased adhesive ability of MSCs after treatment with T cell activation products was dependent on expression of ICAM-1 and VCAM-1. MSC adhesion assay was performed as described in Materials and Methods. A, The MSC-splenocyte cocultures after removal of the nonadhesive lymphocytes are shown (original magnification ×200). Data are representative of three independent experiments. B, Adhesion rates were calculated as the ratio of the number of T cell blasts adhered to MSCs in different treatment groups/group treated with SupCD3-act. Data are means ± SD of a representative of three experiments. **p < 0.01; ***p < 0.001.
FIGURE 4
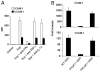
IFN-γ is critical for upregulation of ICAM-1 and VCAM-1. A, MSCs were cultured with SupCD3-act pretreated with Abs to neutralize IFN-γ or TNF-α and IL-1α (20 μg/ml each). After 24 h, cells were analyzed by flow cytometry for expression levels of ICAM-1 and VCAM-1. Data shown are means ± SD of a representative of five experiments. B, MSCs derived from C57BL/6, IFNγR1, and TNFαR1 mice (all in passage five) were treated with SupCD3-act (50% of total volume) for 24 h. The expression of ICAM-1 and VCAM-1 in treated and control MSCs was analyzed by real-time PCR. Then the fold increase in treated MSCs was calculated compared with their respective untreated MSC controls. Data shown are means ± SD of a representative of three experiments.
FIGURE 5
IFN-γ in combination with TNF-α or IL-1 induced the high expression of ICAM-1 and VCAM-1 in MSCs. MSCs were supplemented with recombinant cytokines (20 ng/ml each) for 24 h. The expression of ICAM-1 and VCAM-1 was analyzed by flow cytometry. Data shown are representative of five independent experiments.
FIGURE 6
ICAM-1 and VCAM-1 are critical in MSC-mediated immunosuppression. A, MSCs from _iNOS_−/− or WT C57BL/6 mice were cocultured with fresh C57BL/6 splenocytes plus anti-CD3 for 48 h in the presence or absence of a transwell system (0.4-μm pore membrane, MSCs on the bottom and splenocytes on the top), at a ratio of 1:20 (MSCs to splenocytes). The number indicates the percentage of cells in S+G2/M stages (analyzed by flow cytometry). Data shown are representative of five independent experiments. B, Cocultures of MSCs and SupCD3-act (50% of total volume) were treated with or without anti–ICAM-1 or anti–VCAM-1 (20 μg/ml each). After 24 h, NO production was determined by assaying total nitrates in the culture supernatant using modified Griess reagent. Data shown are means ± SD of a representative of three experiments. C, MSCs were treated first with SupCD3-act (50% of total volume) for 12 h, cocultured with CD4+ T cell blasts plus IL-2 at a 1:20 ratio in the presence of anti–ICAM-1 or anti–VCAM-1 (20 μg/ml each) for 6 h, followed by continued culturing under shaking (100 rpm) in the CO2 incubator at 37°C for 6 h, at the end of which cell proliferation was assessed. Values are means ± SD of five replicate wells from a representative of three experiments. **p < 0.01.
FIGURE 7
ICAM-1–deficient MSCs had a significantly reduced immunosuppressive effect in vitro and in vivo. A, MSCs from ICAM-1–deficient or WT mice were cocultured with fresh splenocytes at different ratios with the addition of soluble anti-CD3 (1 μg/ml) in 96-well plates. Cell proliferation was assayed after 48 h. Values are means ± SD of five replicate wells from a representative of three experiments. B, C57BL/6 mice were immunized with OVA in complete Freund's adjuvant by tail base injection. Mice were challenged in the footpad with 200 μg aggregated OVA administered with or without WT or ICAM-1–deficient MSCs (2.5 × 105 cells) on day 7. Footpad thickness increment was determined after 24 h as a measure of DTH. Data shown are means ± SD of a representative of three experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Similar articles
- Inducible indoleamine 2,3-dioxygenase 1 and programmed death ligand 1 expression as the potency marker for mesenchymal stromal cells.
Guan Q, Li Y, Shpiruk T, Bhagwat S, Wall DA. Guan Q, et al. Cytotherapy. 2018 May;20(5):639-649. doi: 10.1016/j.jcyt.2018.02.003. Epub 2018 Mar 13. Cytotherapy. 2018. PMID: 29548707 - Modulated expression of adhesion molecules and galectin-1: role during mesenchymal stromal cell immunoregulatory functions.
Najar M, Raicevic G, Id Boufker H, Stamatopoulos B, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L. Najar M, et al. Exp Hematol. 2010 Oct;38(10):922-32. doi: 10.1016/j.exphem.2010.05.007. Epub 2010 Jun 1. Exp Hematol. 2010. PMID: 20570633 - Bone marrow mesenchymal stem cells and condition media diminish inflammatory adhesion molecules of pulmonary endothelial cells in an ovalbumin-induced asthmatic rat model.
Rahbarghazi R, Keyhanmanesh R, Aslani MR, Hassanpour M, Ahmadi M. Rahbarghazi R, et al. Microvasc Res. 2019 Jan;121:63-70. doi: 10.1016/j.mvr.2018.10.005. Epub 2018 Oct 18. Microvasc Res. 2019. PMID: 30343002 - Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens.
Li X, Wang Q, Ding L, Wang YX, Zhao ZD, Mao N, Wu CT, Wang H, Zhu H, Ning SB. Li X, et al. Stem Cell Res Ther. 2019 Aug 23;10(1):267. doi: 10.1186/s13287-019-1384-9. Stem Cell Res Ther. 2019. PMID: 31443680 Free PMC article.
Cited by
- Application of mesenchymal stem cells for neurodegenerative diseases therapy discovery.
Trinh QD, Mai HN, Pham DT. Trinh QD, et al. Regen Ther. 2024 Oct 30;26:981-989. doi: 10.1016/j.reth.2024.09.014. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39524179 Free PMC article. Review. - Human dental pulp stem cells modulate pro-inflammatory macrophages both through cell-to-cell contact and paracrine signaling.
Maccaferri M, Pisciotta A, Carnevale G, Salvarani C, Pignatti E. Maccaferri M, et al. Front Immunol. 2024 Oct 10;15:1440974. doi: 10.3389/fimmu.2024.1440974. eCollection 2024. Front Immunol. 2024. PMID: 39450172 Free PMC article. - Mesenchymal stromal cells as cancer promoters.
Antoon R, Overdevest N, Saleh AH, Keating A. Antoon R, et al. Oncogene. 2024 Nov;43(49):3545-3555. doi: 10.1038/s41388-024-03183-1. Epub 2024 Oct 16. Oncogene. 2024. PMID: 39414984 Free PMC article. Review. - Characterization of ibrutinib's effects on the morphology, proliferation, phenotype, viability, and anti-inflammatory potential of adipose-derived mesenchymal stromal cells.
Silva-Carvalho AÉ, Bispo ECI, da Silva IGM, Correa JR, Carvalho JL, Gelfuso GM, Saldanha-Araujo F. Silva-Carvalho AÉ, et al. Sci Rep. 2024 Aug 28;14(1):19906. doi: 10.1038/s41598-024-71054-6. Sci Rep. 2024. PMID: 39191849 Free PMC article. - Therapeutic potential of stem cells in subarachnoid hemorrhage.
Kanamaru H, Suzuki H. Kanamaru H, et al. Neural Regen Res. 2025 Apr 1;20(4):936-945. doi: 10.4103/NRR.NRR-D-24-00124. Epub 2024 May 17. Neural Regen Res. 2025. PMID: 38989928 Free PMC article.
References
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. - PubMed
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. - PubMed
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. - PubMed
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE019413/DE/NIDCR NIH HHS/United States
- R01 DE019413/DE/NIDCR NIH HHS/United States
- R01 DE019413-01/DE/NIDCR NIH HHS/United States
- R01 DE019932-01/DE/NIDCR NIH HHS/United States
- R01 DE019932-02/DE/NIDCR NIH HHS/United States
- R01 DE019413-02/DE/NIDCR NIH HHS/United States
- R01 DE019932/DE/NIDCR NIH HHS/United States
- DE019932/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous