MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells - PubMed (original) (raw)
MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells
Xiaomei Qin et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Endothelial cells (ECs) respond to changes in mechanical forces, leading to the modulation of signaling networks and cell function; an example is the inhibition of EC proliferation by steady laminar flow. MicroRNAs (miRs) are short noncoding 20-22 nucleotide RNAs that negatively regulate the expression of target genes at the posttranscriptional level. This study demonstrates that miRs are involved in the flow regulation of gene expression in ECs. With the use of microRNA chip array, we found that laminar shear stress (12 dyn/cm(2), 12 h) regulated the EC expression of many miRs, including miR-19a. We further showed that stable transfection of miR-19a significantly decreased the expression of a reporter gene controlled by a conserved 3'-untranslated region of the cyclinD1 gene and also the protein level of cyclin D1, leading to an arrest of cell cycle at G1/S transition. Laminar flow suppressed cyclin D1 protein level, and this suppressive effect was diminished when the endogenous miR-19a was inhibited. In conclusion, we demonstrated that miR-19a plays an important role in the flow regulation of cyclin D1 expression. These results revealed a mechanism by which mechanical forces modulate endothelial gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
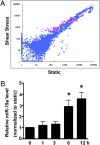
miR-19a is rapidly induced by shear stress in ECs. (A) Scatter plot of miR expression profiles in ECs in response to laminar shear stress (12 dyn/cm2 for 12 h, y axis) and control ECs kept under static condition (x axis). The miRs differentially expressed with statistical significance are marked in red. (B) qRT-PCR shows that miR-19a was induced in ECs after being exposed to laminar shear stress for various time durations. U6 snRNA level was used for normalization. Data are presented as the mean ± SEM (n = 3). *P < 0.05 compared with static control).
Fig. 2.
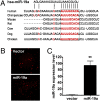
miR-19a represses cyclin D1 expression through a conserved 3′-UTR binding site. (A) Sequence alignment of the miR-19a base-pairing sites in the 3′-UTR of cyclinD1 mRNAs showed that the regions complementary to the 9 nt of miR-19a are highly conserved among human, mouse, rat, cow, dog, and chicken. The “seed” sequences of miR-19a complementary to cyclin D1 are shown in red and boxed. (B) Endothelial cell stable lines were generated by transfecting EA.hy.926 cells with pEF1-RF-miR-19 or pEF-RF and by the puromycin selection. Photomicrographs show ubiquitous expression of red fluorescence protein in both transfected cells. (C) Overexpression of miR-19a was confirmed in the RF-miR-19a stable cell line with the use of qRT-PCR. U6 was used as the internal control. Data are presented as mean ± SEM (n = 3). **P < 0.01.
Fig. 3.
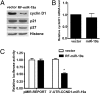
Cyclin D1 is a target of miR-19a in ECs. (A) Western blot analysis showed that protein level of cyclinD1 was decreased in the miR-19a–transfected cell line. (B) Cyclin D1 mRNA was not different between miR-19a–transfected cells and control cells as detected by qRT-PCR. (C) Luciferase activity of the reporter of the cyclin D1 3′-UTR containing miR-19a site (3′-UTR-CCND1-miR19a), but not the pMIR-REPORT control, was decreased in the miR19a-expressing cells. Data are presented as mean ± SEM of three experiments. *P < 0.05 compared with that in the control cell line.
Fig. 4.
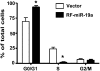
Overexpression of miR-19a arrests cell cycle at the G1 phase in ECs. Flow-cytometric analysis indicated that the population of cells in G0/G1 phase was increased and that in S phase decreased in miR-19a–transfected cells. Data are presented as mean ± SEM of three repeated experiments. *P < 0.05 compared with control cells.
Fig. 5.
miR-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in HUVECs. (A) Effects of laminar flow on the expression of cyclin D1 and p21, another cell cycle–related genes. Confluent HUVECs were exposed to 12 dyn/cm2 shear stress or static control for 12 h and 24 h. Results shown are representative of three independent experiments. (B) Pretreatment with anti–miR-19a prevented the inhibitory effect of laminar flow on cyclin D1 expression. HUVECs were pretreated with anti–miR-19a or control oligonucleotides (negative anti-miR) for 48 h followed by laminar flow for 24 h, and cyclinD1 expression was examined with Western blotting. (C) miR-19a inhibitor partially attenuated the laminar flow-induced cell cycle arrest. HUVECs were transfected with anti–miR-19a or anti-miR negative control for 48 h, followed by 12 dyn/cm2 shear stress or static control for 24 h, and cell cycle distribution of ECs was analyzed with flow cytometry. *P < 0.05 compared with static plus anti-miR negative control. (D) Shear stress–induced suppression of ECs entry S and G2/M phases were reduced by anti–miR-19a. *P < 0.05 compared with anti-miR negative control. Results shown are representative of three independent experiments.
Similar articles
- MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells.
Chen K, Fan W, Wang X, Ke X, Wu G, Hu C. Chen K, et al. Biochem Biophys Res Commun. 2012 Oct 12;427(1):138-42. doi: 10.1016/j.bbrc.2012.09.026. Epub 2012 Sep 16. Biochem Biophys Res Commun. 2012. PMID: 22989749 - MicroRNA-23b regulates cyclin-dependent kinase-activating kinase complex through cyclin H repression to modulate endothelial transcription and growth under flow.
Wang KC, Nguyen P, Weiss A, Yeh YT, Chien HS, Lee A, Teng D, Subramaniam S, Li YS, Chien S. Wang KC, et al. Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1437-45. doi: 10.1161/ATVBAHA.114.303473. Epub 2014 May 22. Arterioscler Thromb Vasc Biol. 2014. PMID: 24855060 Free PMC article. - Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth.
Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, Chien S. Wang KC, et al. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):3234-9. doi: 10.1073/pnas.0914825107. Epub 2010 Jan 27. Proc Natl Acad Sci U S A. 2010. PMID: 20133741 Free PMC article. - MicroRNAs in flow-dependent vascular remodelling.
Neth P, Nazari-Jahantigh M, Schober A, Weber C. Neth P, et al. Cardiovasc Res. 2013 Jul 15;99(2):294-303. doi: 10.1093/cvr/cvt096. Epub 2013 Apr 23. Cardiovasc Res. 2013. PMID: 23612583 Review. - Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs.
Kumar S, Kim CW, Simmons RD, Jo H. Kumar S, et al. Arterioscler Thromb Vasc Biol. 2014 Oct;34(10):2206-16. doi: 10.1161/ATVBAHA.114.303425. Epub 2014 Jul 10. Arterioscler Thromb Vasc Biol. 2014. PMID: 25012134 Free PMC article. Review.
Cited by
- The mechanisms of glycolipid metabolism disorder on vascular injury in type 2 diabetes.
Chen X, Shi C, Wang Y, Yu H, Zhang Y, Zhang J, Li P, Gao J. Chen X, et al. Front Physiol. 2022 Aug 31;13:952445. doi: 10.3389/fphys.2022.952445. eCollection 2022. Front Physiol. 2022. PMID: 36117707 Free PMC article. Review. - Integrated analysis reveals microRNA networks coordinately expressed with key proteins in breast cancer.
Aure MR, Jernström S, Krohn M, Vollan HK, Due EU, Rødland E, Kåresen R; Oslo Breast Cancer Research Consortium (OSBREAC); Ram P, Lu Y, Mills GB, Sahlberg KK, Børresen-Dale AL, Lingjærde OC, Kristensen VN. Aure MR, et al. Genome Med. 2015 Feb 2;7(1):21. doi: 10.1186/s13073-015-0135-5. eCollection 2015. Genome Med. 2015. PMID: 25873999 Free PMC article. - A three-step approach identifies novel shear stress-sensitive endothelial microRNAs involved in vasculoprotective effects of high-intensity interval training (HIIT).
Schmitz B, Breulmann FL, Jubran B, Rolfes F, Thorwesten L, Krüger M, Klose A, Schnittler HJ, Brand SM. Schmitz B, et al. Oncotarget. 2019 Jun 4;10(38):3625-3640. eCollection 2019 Jun 4. Oncotarget. 2019. PMID: 31217898 Free PMC article. - Epigenetic Mechanism in Regulation of Endothelial Function by Disturbed Flow: Induction of DNA Hypermethylation by DNMT1.
Zhou J, Li YS, Wang KC, Chien S. Zhou J, et al. Cell Mol Bioeng. 2014 Jun 1;7(2):218-224. doi: 10.1007/s12195-014-0325-z. Cell Mol Bioeng. 2014. PMID: 24883126 Free PMC article. - Circulating microRNA-19a as a potential novel biomarker for diagnosis of acute myocardial infarction.
Zhong J, He Y, Chen W, Shui X, Chen C, Lei W. Zhong J, et al. Int J Mol Sci. 2014 Nov 6;15(11):20355-64. doi: 10.3390/ijms151120355. Int J Mol Sci. 2014. PMID: 25383678 Free PMC article.
References
- Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. - PubMed
- Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. - PubMed
- Chien S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. - PubMed
- Dekker RJ, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials