Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein - PubMed (original) (raw)
. 2010 Feb 2;107(5):2295-300.
doi: 10.1073/pnas.0911829107. Epub 2010 Jan 19.
Marten Beeg, Matteo Stravalaci, Antonio Bastone, Alessandra Sclip, Emiliano Biasini, Laura Tapella, Laura Colombo, Claudia Manzoni, Tiziana Borsello, Roberto Chiesa, Marco Gobbi, Mario Salmona, Gianluigi Forloni
Affiliations
- PMID: 20133875
- PMCID: PMC2836680
- DOI: 10.1073/pnas.0911829107
Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein
Claudia Balducci et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Inability to form new memories is an early clinical sign of Alzheimer's disease (AD). There is ample evidence that the amyloid-beta (Abeta) peptide plays a key role in the pathogenesis of this disorder. Soluble, bio-derived oligomers of Abeta are proposed as the key mediators of synaptic and cognitive dysfunction, but more tractable models of Abeta-mediated cognitive impairment are needed. Here we report that, in mice, acute intracerebroventricular injections of synthetic Abeta(1-42) oligomers impaired consolidation of the long-term recognition memory, whereas mature Abeta(1-42) fibrils and freshly dissolved peptide did not. The deficit induced by oligomers was reversible and was prevented by an anti-Abeta antibody. It has been suggested that the cellular prion protein (PrP(C)) mediates the impairment of synaptic plasticity induced by Abeta. We confirmed that Abeta(1-42) oligomers interact with PrP(C), with nanomolar affinity. However, PrP-expressing and PrP knock-out mice were equally susceptible to this impairment. These data suggest that Abeta(1-42) oligomers are responsible for cognitive impairment in AD and that PrP(C) is not required.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
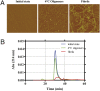
Atomic force microscopy (AFM) and size exclusion chromatography (SEC) of different Aβ1–42 preparations. (A) AFM characterization of the Aβ1–42 preparations used in vivo: the “initial state” corresponds to the freshly dissolved peptide kept at 4 °C; the oligomers were formed after 24 h incubation at 4 °C, pH 7.4, and the fibrils after 24 h of incubation at 37 °C, pH 2 (scan size 2 μm × 2 μm). (B) Initial state (blue), 4 °C Aβ1–42 oligomers (green), and fibrils (red) analyzed by SEC, monitoring absorbance at 214 nm.
Fig. 2.
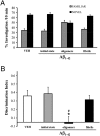
Aβ1–42 oligomers impair recognition memory in mice. (A) Effect of Aβ initial state, oligomers, and fibrils on memory was investigated in C57BL/6 male mice in the object recognition task after two i.c.v. injections (7.5 μL; 1.0 μM). Histograms indicate percentage (mean ± SEM) of exploration of the familiar and novel objects. Vehicle-injected mice (VEH; PBS 5 mM; n = 7) spent significantly more time investigating the novel object. Performance was comparable in mice given initial state Aβ (n = 10) and fibrils (n = 10). The Aβ oligomers significantly impaired memory, as shown by the inability of the mice to recognize the familiar object (n = 13) and spending equal time investigating both objects. (B) Histograms show the corresponding discrimination index (mean ± SEM) for the data shown in A (one-way ANOVA, F 3,36 = 5.76; P = 0.002; *P < 0.05 vs. VEH and fibrils; # P < 0.01 vs. initial state; Tukey’s post hoc test).
Fig. 3.
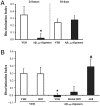
Aβ1–42 oligomer-mediated memory impairment is reversible and is prevented by pretreatment with the anti-Aβ 4G8 antibody. To investigate whether the Aβ oligomer-mediated memory impairment was reversible, mice were injected with oligomers and tested in the object recognition task 24 h or 10 days later. (A) Memory impairment induced by Aβ1–42 oligomers after 24 h (t 12 = -2.34; P = 0.03; *P < 0.05 Student’s t test; n = 7, mean ± SEM) had completely recovered 10 days after the injection (t 12 = 0.48; P = 0.64; Student’s t test). (B) To test whether the deficit was prevented by an anti-Aβ antibody, mice were treated 5 min before Aβ oligomer injection with 0.25 μg of monoclonal antibody 4G8. Analysis of variance indicated a significant interaction (4G8 x Aβ oligomers F 1,20 = 6.5; P = 0.01, ANOVA 2 × 2 test). The antibody alone had no effect, as the memory performance of 4G8-injected mice (n = 5) was comparable to that of vehicle-injected mice (n = 6). Aβ oligomers (n = 6) induced significantly impaired memory (*P < 0.05 vs. VEH or 4G8 alone, Bonferroni’s post hoc test), but this memory impairment was completely rescued by 4G8 pretreatment (n = 7; #P < 0.01 vs. Aβ oligomers, Bonferroni’s post hoc test). Pretreatment with the heat-denatured 4G8 antibody (n = 7) did not restore memory.
Fig. 4.
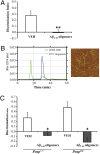
Aβ1–42 oligomers impair recognition memory independently of PrPC. (A) Prnp 0/0 mice given an i.c.v. injection of Aβ oligomers prepared at 4 °C showed significant memory impairment (t 9 = −3,57; **P < 0.01 Student’s t test; VEH n = 5; Aβ1–42 Oligomers n = 6; mean ± SEM). (B) SEC of the 22 °C oligomer preparation (green), initial state (blue). AFM pictures of the oligomeric preparations are shown on the right of the SEC panel (scan size, 2 μm × 2 μm). (C) Oligomeric assemblies prepared at 22 °C significantly affected recognition memory in wild-type mice (Prnp +/+) (t 11 = −2.5; P = 0.03; Student’s t test; VEH n = 6; Aβ1–42 oligomers n = 7) and Prnp 0/0 mice (t 8 = −4.5; P = 0.02; Student’s t test; VEH n = 5; Aβ1–42 oligomers n = 5).
Fig. 5.
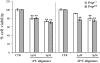
Vulnerability of hippocampal neurons to Aβ1–42 oligomers is independent of PrPC. Histograms show percentage cell survival in MTT test after exposure to 4 °C and 22 °C oligomers (mean ± SEM); 72-h treatment with Aβ1–42 oligomers (1 and 3 μM) caused similar death of hippocampal neurons from Prnp +/+ and Prnp 0/0 mice. Two-way ANOVA for 4 °C oligomers revealed a nonsignificant interaction transgene (tg) × treatment (F 1,12 = 0.29; P = 0.7) and a significant interaction tg × treatment for 22 °C oligomers (F 1,12 = 5.1; P = 0.02), **P < 0.01; Tukey’s test vs. VEH group) .
Fig. 6.
Specific capture of PrPC by 3F4 antibody immobilized on the sensor chip. 3F4 was immobilized on the sensor chip using amine-coupling chemistry, with final immobilization levels of ∼6,000 resonance units, RU. After 90° rotation of the fluid system, brain homogenates from PrPC overexpressing mice or Prnp 0/0 mice were injected in parallel.
Fig. 7.
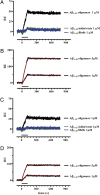
Surface plasmon resonance shows selective, high-affinity binding of Aβ1−42 oligomers to PrPC. The Aβ1–42 species were perfused for 2 min on sensor surfaces on which PrPC had been captured by 3F4 (A and B) or 94B4 (C and D) monoclonal antibodies. The nonspecific binding on sensor surfaces immobilizing the antibodies alone was subtracted. Sensorgrams show the time course of the Aβ−dependent SPR signal in resonance units (RU). Only Aβ oligomers bound PrPC specifically, whereas the initial state and fibrils did not (A and C). The sensorgrams obtained with 1- and 5-μM Aβ1−42 oligomers were analyzed by the Langmuir equation, modeling a simple bimolecular interaction (B and D). Fitting is shown in red. Parameters of Aβ oligomer binding to (3F4)-PrPC were as follows: K on: 2.1 × 103 M-1s−1; K off: 4.0 × 10−5 s−1; K d: 19.5 nM; Rmax: 211 RU; for binding to (94B4)-PrPC: K on: 1.8 × 103 M-1s−1; K off: 4.0 × 10−5 s−1; K d: 22.6 nM; Rmax: 143 RU.
Fig. 8.
Aβ oligomers acutely disrupt memory storage but not memory retrieval. To clarify the Aβ oligomers’ action on memory formation and recall, mice were given a single i.c.v. injection of oligomers either before familiarization or before memory recall evaluation. One-way ANOVA revealed a significant effect of treatment (F 2,22 = 7.05; P = 0.043). The memory impairment was observed only in animals receiving Aβ oligomers before the familiarization phase (prefamiliarization; n = 10), which were unable to distinguish between the two objects (*P < 0.05 vs. VEH; # P < 0.01 vs. oligomers prerecall; Tukey’s posthoc test). No effect was detectable when mice were treated with either vehicle (n = 8) or oligomers before memory recall evaluation (oligomer prerecall; n = 7).
Similar articles
- Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers.
Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Laurén J, et al. Nature. 2009 Feb 26;457(7233):1128-32. doi: 10.1038/nature07761. Nature. 2009. PMID: 19242475 Free PMC article. - Soluble prion protein and its N-terminal fragment prevent impairment of synaptic plasticity by Aβ oligomers: Implications for novel therapeutic strategy in Alzheimer's disease.
Scott-McKean JJ, Surewicz K, Choi JK, Ruffin VA, Salameh AI, Nieznanski K, Costa ACS, Surewicz WK. Scott-McKean JJ, et al. Neurobiol Dis. 2016 Jul;91:124-131. doi: 10.1016/j.nbd.2016.03.001. Epub 2016 Mar 3. Neurobiol Dis. 2016. PMID: 26949218 Free PMC article. - β-amyloid oligomers and prion protein: Fatal attraction?
Forloni G, Balducci C. Forloni G, et al. Prion. 2011 Jan-Mar;5(1):10-5. doi: 10.4161/pri.5.1.14367. Epub 2011 Jan 1. Prion. 2011. PMID: 21150333 Free PMC article. - In vivo application of beta amyloid oligomers: a simple tool to evaluate mechanisms of action and new therapeutic approaches.
Balducci C, Forloni G. Balducci C, et al. Curr Pharm Des. 2014;20(15):2491-505. doi: 10.2174/13816128113199990497. Curr Pharm Des. 2014. PMID: 23859553 Review. - Cellular prion protein mediates the toxicity of beta-amyloid oligomers: implications for Alzheimer disease.
Nygaard HB, Strittmatter SM. Nygaard HB, et al. Arch Neurol. 2009 Nov;66(11):1325-8. doi: 10.1001/archneurol.2009.223. Arch Neurol. 2009. PMID: 19901162 Free PMC article. Review.
Cited by
- Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease.
Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. Busche MA, et al. Proc Natl Acad Sci U S A. 2012 May 29;109(22):8740-5. doi: 10.1073/pnas.1206171109. Epub 2012 May 16. Proc Natl Acad Sci U S A. 2012. PMID: 22592800 Free PMC article. - Neurotoxicity of amyloid β-protein: synaptic and network dysfunction.
Mucke L, Selkoe DJ. Mucke L, et al. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006338. doi: 10.1101/cshperspect.a006338. Cold Spring Harb Perspect Med. 2012. PMID: 22762015 Free PMC article. Review. - Differential regulation of amyloid-β endocytic trafficking and lysosomal degradation by apolipoprotein E isoforms.
Li J, Kanekiyo T, Shinohara M, Zhang Y, LaDu MJ, Xu H, Bu G. Li J, et al. J Biol Chem. 2012 Dec 28;287(53):44593-601. doi: 10.1074/jbc.M112.420224. Epub 2012 Nov 6. J Biol Chem. 2012. PMID: 23132858 Free PMC article. - Proteolytic processing of the prion protein in health and disease.
Altmeppen HC, Puig B, Dohler F, Thurm DK, Falker C, Krasemann S, Glatzel M. Altmeppen HC, et al. Am J Neurodegener Dis. 2012;1(1):15-31. Epub 2012 May 15. Am J Neurodegener Dis. 2012. PMID: 23383379 Free PMC article. - FcγRIIb mediates amyloid-β neurotoxicity and memory impairment in Alzheimer's disease.
Kam TI, Song S, Gwon Y, Park H, Yan JJ, Im I, Choi JW, Choi TY, Kim J, Song DK, Takai T, Kim YC, Kim KS, Choi SY, Choi S, Klein WL, Yuan J, Jung YK. Kam TI, et al. J Clin Invest. 2013 Jul;123(7):2791-802. doi: 10.1172/JCI66827. Epub 2013 Jun 10. J Clin Invest. 2013. PMID: 23921129 Free PMC article.
References
- McLean CA, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials