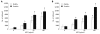Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion - PubMed (original) (raw)
Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion
Apaporn Menakongka et al. World J Gastroenterol. 2010.
Abstract
Aim: To investigate the role of hepatocyte growth factor (HGF) in cholangiocarcinoma (CCA) cell invasiveness and the mechanisms underlying such cellular responses.
Methods: Effects of HGF on cell invasion and motility were investigated in two human CCA cell lines, HuCCA-1 and KKU-M213, using Transwell in vitro assay. Levels of proteins of interest and their phosphorylated forms were determined by Western blotting. Localization of E-cadherin was analyzed by immunofluorescence staining and visualized under confocal microscope. Activities of matrix degrading enzymes were determined by zymography.
Results: Both CCA cell lines expressed higher Met levels than the H69 immortalized cholangiocyte cell line. HGF induced invasion and motility of the cell lines and altered E-cadherin from membrane to cytoplasm localization, but did not affect the levels of secreted matrix metalloproteinase (MMP)-2, MMP-9 and urokinase plasminogen activator, key matrix degrading enzymes involved in cell invasion. Concomitantly, HGF stimulated Akt and extracellular signal-regulated kinase (ERK)1/2 phosphorylation but with slightly different kinetic profiles in the two cell lines. Inhibition of the phosphoinositide 3-kinase (PI3K)/Akt pathway by the PI3K inhibitor, LY294002, markedly suppressed HGF-stimulated invasion of both CCA cell lines, and inhibition of the ERK pathway by U0126 suppressed HGF-induced invasion of the KKU-M213 cell line but had a moderate effect on HuCCA-1 cells.
Conclusion: These data indicate that HGF promotes CCA cell invasiveness through dys-localization of E-cadherin and induction of cell motility by distinct signaling pathways depending on cell line type.
Figures
Figure 1
Steady state level of Met expression in cholangiocarcinoma cell lines and activation by hepatocyte growth factor (HGF). Cell lysates from 80% confluent cells cultured in 10% fetal bovine serum (FBS) medium were examined for Met expression by Western blotting analysis (A). Lysates from HuCCA-1 (B) and KKU-M213 (C) cells treated with or without 50 ng/mL HGF for various times were analyzed by Western blotting for levels of Met and phospho-Met (pY1234/1235). The graphs show band densities of phospho-Met relative to those at zero time points. Data are presented as mean ± SE of results obtained from three independent experiments. a_P_ < 0.05 vs untreated control.
Figure 2
HGF induction of cholangiocarcinoma motility and invasiveness. In vitro invasion and motility assays of HuCCA-1 (A) and KKU-M213 (B) cells were conducted in a Transwell unit coated with and without Matrigel. Cells (105) in serum-free medium were plated in the upper chamber of a Transwell unit and 0-100 ng/mL HGF added to the lower chamber. After 6 h of incubation, cells invading to the lower compartment of the Transwell unit were stained and counted. The numbers of invaded/motile cells are presented as mean ± SE of results obtained from three independent experiments. a_P_ < 0.05, b_P_ < 0.01, vs untreated control.
Figure 3
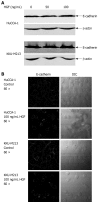
Effects of HGF on E-cadherin expression and localization. A: cholangiocarcinoma (CCA) cells were treated with HGF for 6 h, then cell lysate was analyzed by Western blotting with anti-E-cadherin and -β-actin monoclonal antibodies; B: After treatment with 0 and 100 ng/mL HGF for 6 h, cells were analyzed by immunofluorescence using anti-E-cadherin antibody and visualized under confocal laser scanning microscopy (60 × objective magnification plus 2 × digital magnification).
Figure 4
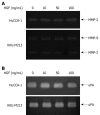
Effect of HGF on levels of secreted matrix degrading enzymes from cholangiocarcinoma HuCCA-1 and KKU-M213 cell lines. Cells were treated with various concentrations of HGF (0-100 ng/mL) in serum-free medium for 6 h. Conditioned media were then analyzed for MMP-2 (approximate 65 kDa) and MMP-9 (approximate 85 kDa) gelatinolytic activity by gelatin zymography (A) and for uPA by plasminogen-gelatin zymography (B).
Figure 5
HGF induction of ERK1/2 and Akt phosphorylation in cholangiocarcinoma HuCCA-1 and KKU-M213 cell lines. About 80% confluent cells were treated with 50 ng/mL HGF in serum-free medium for 15, 60, 360 min. Lysates from HuCCA-1 (A) and KKU-M213 (B) cells were assessed for total and phosphorylated forms of ERK1/2 and Akt by Western blotting assay. The graphs showed band densities of phospho-ERK1/2 and phospho-Akt relative to those at zero time points. Data are presented as mean ± SE of results obtained from three independent experiments. a_P_ < 0.05 vs untreated control.
Figure 6
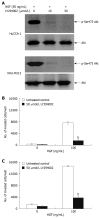
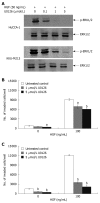
Suppression of HGF-induced cholangiocarcinoma cell invasiveness by PI3-kinase inhibitor, LY294002. HuCCA-1 and KKU-M213 cells were treated with 50 ng/mL HGF in the absence (control) or presence of 10 and 50 μmol/L LY294002 for 6 h, and subsequently Akt phosphorylation was determined by Western blotting (A). In vitro invasion of HuCCA-1 (B) and KKU-M213 (C) cells was evaluated in the absence or presence of HGF with or without 50 μmol/L LY294002. Numbers of invaded cells are presented as mean ± SE of results obtained from three independent experiments. b_P_ < 0.01 vs control.
Figure 7
Suppression of HGF-induced cholangiocarcinoma cell invasiveness by MEK1 inhibitor, U0126. HuCCA-1 and KKU-M213 cells were treated with 50 ng/mL HGF in the absence (control) or presence of 0.1, 1 and 5 μmol/L U0126 for 6 h, and subsequently ERK1/2 phosphorylation was determined by Western blotting (A). In vitro invasion of HuCCA-1 (B) and KKU-M213 (C) cells was evaluated in the absence or presence of HGF with or without 1 and 5 μmol/L U0126. Numbers of invaded cells are presented as mean ± SE of results obtained from three independent experiments. a_P_ < 0.05 and b_P_ < 0.01 vs control.
Similar articles
- High level of urokinase plasminogen activator contributes to cholangiocarcinoma invasion and metastasis.
Thummarati P, Wijitburaphat S, Prasopthum A, Menakongka A, Sripa B, Tohtong R, Suthiphongchai T. Thummarati P, et al. World J Gastroenterol. 2012 Jan 21;18(3):244-50. doi: 10.3748/wjg.v18.i3.244. World J Gastroenterol. 2012. PMID: 22294827 Free PMC article. - Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy.
Yothaisong S, Dokduang H, Techasen A, Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ, Loilome W. Yothaisong S, et al. Tumour Biol. 2013 Dec;34(6):3637-48. doi: 10.1007/s13277-013-0945-2. Epub 2013 Jul 6. Tumour Biol. 2013. PMID: 23832540 - Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met.
Leelawat K, Leelawat S, Tepaksorn P, Rattanasinganchan P, Leungchaweng A, Tohtong R, Sobhon P. Leelawat K, et al. J Surg Res. 2006 Nov;136(1):78-84. doi: 10.1016/j.jss.2006.05.031. Epub 2006 Sep 1. J Surg Res. 2006. PMID: 16950403 - Pathogenesis, diagnosis, and management of cholangiocarcinoma.
Ilyas SI, Gores GJ. Ilyas SI, et al. Gastroenterology. 2013 Dec;145(6):1215-29. doi: 10.1053/j.gastro.2013.10.013. Epub 2013 Oct 15. Gastroenterology. 2013. PMID: 24140396 Free PMC article. Review. - Molecular Targets and Signaling Pathways in Cholangiocarcinoma: A Systematic Review.
Idris R, Chaijaroenkul W, Na-Bangchang K. Idris R, et al. Asian Pac J Cancer Prev. 2023 Mar 1;24(3):741-751. doi: 10.31557/APJCP.2023.24.3.741. Asian Pac J Cancer Prev. 2023. PMID: 36974526 Free PMC article.
Cited by
- IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism.
Yamada D, Ilyas SI, Razumilava N, Bronk SF, Davila JI, Champion MD, Borad MJ, Bezerra JA, Chen X, Gores GJ. Yamada D, et al. Hepatology. 2015 May;61(5):1627-42. doi: 10.1002/hep.27687. Epub 2015 Mar 20. Hepatology. 2015. PMID: 25580681 Free PMC article. - Transforming Growth Factor-Beta (TGFβ) Signaling Pathway in Cholangiocarcinoma.
Papoutsoglou P, Louis C, Coulouarn C. Papoutsoglou P, et al. Cells. 2019 Aug 23;8(9):960. doi: 10.3390/cells8090960. Cells. 2019. PMID: 31450767 Free PMC article. Review. - FGF-1/-3/FGFR4 signaling in cancer-associated fibroblasts promotes tumor progression in colon cancer through Erk and MMP-7.
Bai YP, Shang K, Chen H, Ding F, Wang Z, Liang C, Xu Y, Sun MH, Li YY. Bai YP, et al. Cancer Sci. 2015 Oct;106(10):1278-87. doi: 10.1111/cas.12745. Epub 2015 Sep 4. Cancer Sci. 2015. PMID: 26183471 Free PMC article. - Ceruloplasmin as a prognostic marker in patients with bile duct cancer.
Han IW, Jang JY, Kwon W, Park T, Kim Y, Lee KB, Kim SW. Han IW, et al. Oncotarget. 2017 Apr 25;8(17):29028-29037. doi: 10.18632/oncotarget.15995. Oncotarget. 2017. PMID: 28423673 Free PMC article. - Effects of AKT inhibition on HGF-mediated erlotinib resistance in non-small cell lung cancer cell lines.
Holland WS, Chinn DC, Lara PN Jr, Gandara DR, Mack PC. Holland WS, et al. J Cancer Res Clin Oncol. 2015 Apr;141(4):615-26. doi: 10.1007/s00432-014-1855-4. Epub 2014 Oct 17. J Cancer Res Clin Oncol. 2015. PMID: 25323938 Free PMC article.
References
- Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. - PubMed
- Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33–42. - PubMed
- Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16:363–372. - PubMed
- Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous