Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization - PubMed (original) (raw)
Comparative Study
Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization
Christina L Sherry et al. Brain Behav Immun. 2010 May.
Abstract
Peripheral activation of the immune system by infectious agents triggers the brain-cytokine system causing sickness behaviors which profoundly impact well-being. Dietary fiber is a beneficial foodstuff that, from a gastrointestinal tract perspective, exists in both insoluble and soluble forms. We show that a diet rich in soluble fiber protects mice from endotoxin-induced sickness behavior by polarizing mice Th2 when compared to a diet containing only insoluble fiber. Mice fed soluble fiber became less sick and recovered faster from endotoxin-induced sickness behaviors than mice fed insoluble fiber. In response to intraperitoneal endotoxin, mice fed soluble fiber had up-regulated IL-1RA and reduced IL-1beta and TNF-alpha in the brain as compared to mice fed insoluble fiber. Importantly, mice fed soluble fiber had a basal increase in IL-4 in the ileum and spleen which was absent in MyD88 knockout mice. Con-A stimulated splenocytes from mice fed soluble fiber showed increased IL-4 and IL-5 and decreased IL-2, IL-12 and IFN-gamma when compared to mice fed insoluble fiber. Likewise, endotoxin-stimulated macrophages from mice fed soluble fiber demonstrated decreased IL-1beta, TNF-alpha, IFN-gamma, IL-12 and nitrate and increased IL-1RA, arginase 1 and Ym1 when compared to mice fed insoluble fiber. Finally, the behavioral protection afforded by feeding mice soluble fiber was reduced in IL-4 knockout mice, as was the impact of soluble fiber on Con-A stimulated splenocytes and endotoxin activated macrophages. These data show that a diet rich in soluble fiber protects against endotoxin-induced sickness behavior by polarizing mice Th2 and promoting alternative activation of macrophages.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Fig. 1. Soluble fiber protects against endotoxemia
A, Chow and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. B, 10% pectin (pectin) and cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. C, Pectin and cellulose fed mice were administered LPS IP (0 h) and temperature measured. Results are expressed as a change from pre-LPS (0 h) measurement, means ± SEM; n = 4; arrowp<0.05, main effect of time. D, Pectin and cellulose fed mice were administered LPS IP and food intake and body weight measured 24 h post LPS. Results are expressed as percentage change from the pre-LPS measurement, means ± SEM; n = 4; *p<0.05, main effect of diet. E, Pectin and 10% cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS
Fig. 1. Soluble fiber protects against endotoxemia
A, Chow and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. B, 10% pectin (pectin) and cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. C, Pectin and cellulose fed mice were administered LPS IP (0 h) and temperature measured. Results are expressed as a change from pre-LPS (0 h) measurement, means ± SEM; n = 4; arrowp<0.05, main effect of time. D, Pectin and cellulose fed mice were administered LPS IP and food intake and body weight measured 24 h post LPS. Results are expressed as percentage change from the pre-LPS measurement, means ± SEM; n = 4; *p<0.05, main effect of diet. E, Pectin and 10% cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS
Fig. 1. Soluble fiber protects against endotoxemia
A, Chow and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. B, 10% pectin (pectin) and cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. C, Pectin and cellulose fed mice were administered LPS IP (0 h) and temperature measured. Results are expressed as a change from pre-LPS (0 h) measurement, means ± SEM; n = 4; arrowp<0.05, main effect of time. D, Pectin and cellulose fed mice were administered LPS IP and food intake and body weight measured 24 h post LPS. Results are expressed as percentage change from the pre-LPS measurement, means ± SEM; n = 4; *p<0.05, main effect of diet. E, Pectin and 10% cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS
Fig. 1. Soluble fiber protects against endotoxemia
A, Chow and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. B, 10% pectin (pectin) and cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. C, Pectin and cellulose fed mice were administered LPS IP (0 h) and temperature measured. Results are expressed as a change from pre-LPS (0 h) measurement, means ± SEM; n = 4; arrowp<0.05, main effect of time. D, Pectin and cellulose fed mice were administered LPS IP and food intake and body weight measured 24 h post LPS. Results are expressed as percentage change from the pre-LPS measurement, means ± SEM; n = 4; *p<0.05, main effect of diet. E, Pectin and 10% cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS
Fig. 1. Soluble fiber protects against endotoxemia
A, Chow and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. B, 10% pectin (pectin) and cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. C, Pectin and cellulose fed mice were administered LPS IP (0 h) and temperature measured. Results are expressed as a change from pre-LPS (0 h) measurement, means ± SEM; n = 4; arrowp<0.05, main effect of time. D, Pectin and cellulose fed mice were administered LPS IP and food intake and body weight measured 24 h post LPS. Results are expressed as percentage change from the pre-LPS measurement, means ± SEM; n = 4; *p<0.05, main effect of diet. E, Pectin and 10% cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS
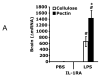
Fig. 2. Soluble fiber up-regulates IL-1RA
10% pectin (pectin) and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP. At 2 h post LPS, real-time RT-PCR was used to quantify IL-1RA (A), IL-1β(B), TNF-α(B) and IL-6 (B) mRNAs from brain. Results are expressed as relative change in mRNA expression ( mRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet, #p<0.05 treatment effect of LPS.
Fig. 2. Soluble fiber up-regulates IL-1RA
10% pectin (pectin) and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP. At 2 h post LPS, real-time RT-PCR was used to quantify IL-1RA (A), IL-1β(B), TNF-α(B) and IL-6 (B) mRNAs from brain. Results are expressed as relative change in mRNA expression ( mRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet, #p<0.05 treatment effect of LPS.
Fig. 3. IL-4 production is augmented by soluble fiber
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. A, Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet. B, ELISA was used to quantify IL-4 from the indicated tissues. Results are expressed as means ± SEM; n = 5-7; *p<0.05 main effect of diet. C, Wild type (WT) and MyD88 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 3; *p<0.05 main effect of diet.
Fig. 3. IL-4 production is augmented by soluble fiber
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. A, Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet. B, ELISA was used to quantify IL-4 from the indicated tissues. Results are expressed as means ± SEM; n = 5-7; *p<0.05 main effect of diet. C, Wild type (WT) and MyD88 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 3; *p<0.05 main effect of diet.
Fig. 3. IL-4 production is augmented by soluble fiber
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. A, Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet. B, ELISA was used to quantify IL-4 from the indicated tissues. Results are expressed as means ± SEM; n = 5-7; *p<0.05 main effect of diet. C, Wild type (WT) and MyD88 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 3; *p<0.05 main effect of diet.
Fig. 4. Arginase and Ym1 expression in peritoneal macrophages
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify arginase (Arg) and Ym1 mRNAs from peritoneal macrophages. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet.
Fig. 5. IL-4 knockout (KO) blunts the impact of soluble fiber on endotoxemia resistance
A, 10% pectin (pectin) and 5% cellulose (cellulose) fed wild type (WT) and IL-4 KO mice were administered carrier (PBS) or LPS IP and social withdrawal measured at 2 h post LPS. Results are expressed as percentage change from cellulose PBS WT mice, means ± SEM; n = 3-4; #p<0.05, treatment effect of LPS, *p<0.05 main effect of diet, $p<0.05 phenotype effect. B, IL-4 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify arginase (Arg) and Ym1 mRNAs from peritoneal macrophages. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 3; *p<0.05 main effect of diet.
Fig. 5. IL-4 knockout (KO) blunts the impact of soluble fiber on endotoxemia resistance
A, 10% pectin (pectin) and 5% cellulose (cellulose) fed wild type (WT) and IL-4 KO mice were administered carrier (PBS) or LPS IP and social withdrawal measured at 2 h post LPS. Results are expressed as percentage change from cellulose PBS WT mice, means ± SEM; n = 3-4; #p<0.05, treatment effect of LPS, *p<0.05 main effect of diet, $p<0.05 phenotype effect. B, IL-4 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify arginase (Arg) and Ym1 mRNAs from peritoneal macrophages. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 3; *p<0.05 main effect of diet.
Similar articles
- Dietary fatty acids influence the production of Th1- but not Th2-type cytokines.
Wallace FA, Miles EA, Evans C, Stock TE, Yaqoob P, Calder PC. Wallace FA, et al. J Leukoc Biol. 2001 Mar;69(3):449-57. J Leukoc Biol. 2001. PMID: 11261793 - Brucella abortus phosphoglyceromutase and dihydrodipicolinate reductase induce Th1 and Th2-related immune responses.
Li Z, Zhang H, Zhang J, Xi L, Yang G, Wang S, Zhou Q, Zhang X, Zhang J. Li Z, et al. World J Microbiol Biotechnol. 2018 Jan 4;34(2):22. doi: 10.1007/s11274-017-2405-4. World J Microbiol Biotechnol. 2018. PMID: 29302824 - The 1996 Moyer Award. Effects of endotoxin on the Th1/Th2 response in humans.
Zimmer S, Pollard V, Marshall GD, Garofalo RP, Traber D, Prough D, Herndon DN. Zimmer S, et al. J Burn Care Rehabil. 1996 Nov-Dec;17(6 Pt 1):491-6. J Burn Care Rehabil. 1996. PMID: 8951535 Review. - Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.
de Lima JD, de Paula AGP, Yuasa BS, de Souza Smanioto CC, da Cruz Silva MC, Dos Santos PI, Prado KB, Winter Boldt AB, Braga TT. de Lima JD, et al. Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
- Mutual Metabolic Interactions in Co-cultures of the Intestinal Anaerostipes rhamnosivorans With an Acetogen, Methanogen, or Pectin-Degrader Affecting Butyrate Production.
Bui TPN, Schols HA, Jonathan M, Stams AJM, de Vos WM, Plugge CM. Bui TPN, et al. Front Microbiol. 2019 Nov 1;10:2449. doi: 10.3389/fmicb.2019.02449. eCollection 2019. Front Microbiol. 2019. PMID: 31736896 Free PMC article. - The relation of saturated fats and dietary cholesterol to childhood cognitive flexibility.
Khan NA, Raine LB, Drollette ES, Scudder MR, Hillman CH. Khan NA, et al. Appetite. 2015 Oct;93:51-6. doi: 10.1016/j.appet.2015.04.012. Epub 2015 Apr 9. Appetite. 2015. PMID: 25865659 Free PMC article. - Sugar-sweetened beverage but not diluted cloudy apple juice consumption induces post-prandial endotoxemia in healthy adults.
Staltner R, Valder S, Wodak MF, Köpsel M, Herdegen V, Esatbeyoglu T, Kostov T, Diel P, Bergheim I. Staltner R, et al. NPJ Sci Food. 2024 Jun 21;8(1):38. doi: 10.1038/s41538-024-00283-w. NPJ Sci Food. 2024. PMID: 38906893 Free PMC article. - Fruit and Juice Epigenetic Signatures Are Associated with Independent Immunoregulatory Pathways.
Nicodemus-Johnson J, Sinnott RA. Nicodemus-Johnson J, et al. Nutrients. 2017 Jul 14;9(7):752. doi: 10.3390/nu9070752. Nutrients. 2017. PMID: 28708104 Free PMC article. - Relationship between fruit and vegetable intake and interference control in breast cancer survivors.
Zuniga KE, Mackenzie MJ, Roberts SA, Raine LB, Hillman CH, Kramer AF, McAuley E. Zuniga KE, et al. Eur J Nutr. 2016 Jun;55(4):1555-62. doi: 10.1007/s00394-015-0973-3. Epub 2015 Jun 30. Eur J Nutr. 2016. PMID: 26123915
References
- Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. - PubMed
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. - PubMed
- Bowen H, Kelly A, Lee T, Lavender P. Control of cytokine gene transcription in Th1 and Th2 cells. Clin Exp Allergy. 2008;38:1422–1431. - PubMed
- Burgess W, Gheusi G, Yao J, Johnson RW, Dantzer R, Kelley KW. Interleukin-1beta-converting enzyme-deficient mice resist central but not systemic endotoxin-induced anorexia. Am J Physiol. 1998;274:R1829–33. - PubMed
- Cavaglieri CR, Nishiyama A, Fernandes LC, Curi R, Miles EA, Calder PC. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS058525-02/NS/NINDS NIH HHS/United States
- R01 DK064862-06/DK/NIDDK NIH HHS/United States
- 5T32 DK059802/DK/NIDDK NIH HHS/United States
- AA019357/AA/NIAAA NIH HHS/United States
- T32 DK059802/DK/NIDDK NIH HHS/United States
- R01 DK064862/DK/NIDDK NIH HHS/United States
- RC1 AA019357-01/AA/NIAAA NIH HHS/United States
- DK064862/DK/NIDDK NIH HHS/United States
- RC1 AA019357/AA/NIAAA NIH HHS/United States
- NS058525/NS/NINDS NIH HHS/United States
- R01 NS058525/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous