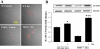Inhibition of telomerase activity alters tight junction protein expression and induces transendothelial migration of HIV-1-infected cells - PubMed (original) (raw)
Inhibition of telomerase activity alters tight junction protein expression and induces transendothelial migration of HIV-1-infected cells
Wen Huang et al. Am J Physiol Heart Circ Physiol. 2010 Apr.
Abstract
Telomerase, via its catalytic component telomerase reverse transcriptase (TERT), extends telomeres of eukaryotic chromosomes. The importance of this reaction is related to the fact that telomere shortening is a rate-limiting mechanism for human life span that induces cell senescence and contributes to the development of age-related pathologies. The aim of the present study was to evaluate whether the modulation of telomerase activity can influence human immunodeficiency virus type 1 (HIV-1)-mediated dysfunction of human brain endothelial cells (hCMEC/D3 cells) and transendothelial migration of HIV-1-infected cells. Telomerase activity was modulated in hCMEC/D3 cells via small interfering RNA-targeting human TERT (hTERT) or by using a specific pharmacological inhibitor of telomerase, TAG-6. The inhibition of hTERT resulted in the upregulation of HIV-1-induced overexpression of intercellular adhesion molecule-1 via the nuclear factor-kappaB-regulated mechanism and induced the transendothelial migration of HIV-1-infected monocytic U937 cells. In addition, the blocking of hTERT activity potentiated a HIV-induced downregulation of the expression of tight junction proteins. These results were confirmed in TERT-deficient mice injected with HIV-1-specific protein Tat into the cerebral vasculature. Further studies revealed that the upregulation of matrix metalloproteinase-9 is the underlying mechanisms of disruption of tight junction proteins in hCMEC/D3 cells with inhibited TERT and exposed to HIV-1. These results indicate that the senescence of brain endothelial cells may predispose to the HIV-induced upregulation of inflammatory mediators and the disruption of the barrier function at the level of the brain endothelium.
Figures
Fig. 1.
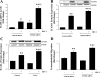
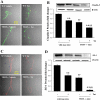
Human telomerase reverse transcriptase (hTERT) silencing results in senescence of hCMEC/D3 cells. Confluent hCMEC/D3 cells cultured on 6-well plates were exposed to 1.5 × 106 HIV-1-infected monocytic U937 cells for 24 h. Controls were exposed to the same amount of uninfected U937 cells. Silencing was performed by transfection of hCMEC/D3 cells with hTERT-specific or control siRNA for 5 h. A: the effects of HIV-1 exposure and/or hTERT silencing on hTERT protein expression was analyzed by Western blot analysis. B: telomerase activity was measured by telomeric repeat amplification protocol assay, and the amplification products were detected by a fluorescence plate reader. TPG, total product generated. C: cellular senescence was analyzed by the assessment of β-galactosidase activity by histochemistry. Cells positive for β-galactosidase activity are stained blue (arrows). Results are means ± SE of 4 separate experiments. *P < 0.05 or **P < 0.01, data in cultures exposed to HIV-1 are significantly different from the corresponding controls without HIV treatment. †P < 0.05 or ††P < 0.01, data in control [nonexposed to human immunodeficiency virus type 1 (HIV-1)] cultures transfected with hTERT small interfering RNA (siRNA) are significantly different from the corresponding control cultures transfected with control siRNA. #P < 0.05, data in cultures transfected with hTERT siRNA and treated with HIV-1 are significantly different from the corresponding cultures transfected with control siRNA and exposed to HIV-1.
Fig. 2.
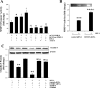
Inhibition of hTERT potentiates HIV-induced overexpression of ICAM-1 in hCMEC/D3 cells. A and B: hCMEC/D3 cultures were transfected with hTERT or control siRNA and exposed to HIV-1-infected or uninfected monocytes as in Fig. 1. In selected experiments (C), cultures were pretreated with telomerase inhibitor, TAG-6 (2 μM), for 2 h instead of transfection with hTERT siRNA. ICAM-1 mRNA (A) and protein (B and C) were determined by real-time RT-PCR and Western blot analysis, respectively. The blots in B and C are representative images from 3 independent experiments, and the quantified results are depicted in the bar graphs. D: inhibition of hTERT potentiates HIV-induced monocyte migration across the in vitro model of the blood-brain barrier. The blood-brain barrier model consisted of cocultures of hCMEC/D3 cells with astrocytes plated in upper and lower chambers, respectively, of the Transwell system. hCMEC/D3 cells were transfected with hTERT or control siRNA as in Fig. 1. HIV-1-infected or uninfected U937 cells were labeled with calcein-AM, added to the upper chamber of the Transwell system in the amount of 1 × 106, and allowed to migrate across hCMEC/D3 monolayers for 24 h. Results are means ± SE of 4 separate experiments. *P < 0.05 or **P < 0.01, data in cultures exposed to HIV-1 are significantly different from the corresponding controls without HIV treatment. †P < 0.05, data in cultures with inhibited TERT activity by hTERT siRNA or TAG-6 and not exposed to HIV are significantly different from the corresponding control cultures without TERT inhibition. #P < 0.05 or ##P < 0.01, data in cultures with inhibited TERT by hTERT siRNA or TAG-6 and treated with HIV-1 are significantly different from the corresponding cultures without TERT inhibition and exposed to HIV-1.
Fig. 3.
HIV Tat-induced overexpression of ICAM-1 is potentiated in brain microvessels of TERT-deficient (TERT−/−) mice. Tat protein (50 μg/mouse) was administered into the internal carotid artery of TERT−/− or wild-type (WT) mice. Brain microvessels were isolated 24 h post-Tat injection and analyzed for ICAM-1 using immunofluorescence (A) and Western blot analysis (B). Control mice were injected with vehicle. The immunostaining data are merged immunofluorescence and phase-contrast micrographs acquired using a ×60 oil-immersion lens under identical instrument settings. Western blot analyses were preformed on isolated microvessels. The blots are representative images from 3 mice per group, and the quantified results are depicted in the bar graphs. Results are means ± SE. *P < 0.05 or **P < 0.01, data in WT or TERT−/− mice injected with Tat are significantly different from the corresponding controls injected with vehicle. ##P < 0.01, data in TERT−/− mice treated with Tat are significantly different from the corresponding WT mice injected with Tat.
Fig. 4.
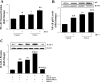
NF-κB regulates ICAM-1 overexpression induced by telomerase inhibition and HIV-1- exposure. A: NF-κB transactivation was determined by luciferase activity in hCMEC/D3 cells upon transfection with the pNF-κB-Luc construct and treatment with HIV-1 particles (200 pg p24/ml) for 24 h. Cotransfection with the control pRL-TK construct allowed normalization of the obtained results to renilla luciferase. Cells were pretreated with TAG-6 (2 μM) 2 h before HIV-1 exposure. B: NF-κB p65 levels were determined in nuclear fraction of hCMEC/D3 cells pretreated with TAG-6 (2 μM; 2 h before HIV-1 exposure) and exposed to 1.5 × 106 HIV-1-infected or uninfected U937 cells for 24 h. C: ICAM-1 protein expression was determined by Western blot analysis in cells transfected with hTERT and exposed to HIV-1-infected U937 cells as in Fig. 1. Selected cultures were pretreated with 4-methyl-N1_-(3-phenylpropyl)benzene-1,2-diamine (JSH-23) (30 μM) 2 h before HIV-1 exposure. The blots in B and C are representative images from 3 independent experiments, and the quantified results are depicted in bar graphs. Results are means ± SE of 3 experiments. *P < 0.05 or **P < 0.01, data in cultures exposed to HIV-1 are significantly different from the corresponding controls without HIV treatment. †_P < 0.05 or ††P < 0.01, data in cultures with inhibited TERT activity by hTERT siRNA or TAG-6 and not exposed to HIV are significantly different from the corresponding control cultures without TERT inhibition. #P < 0.05 or ##P < 0.01, data in cultures with inhibited TERT activity by hTERT siRNA or TAG-6 and treated with HIV-1 are significantly different from the corresponding cultures without TERT inhibition and exposed to HIV-1. ‡‡P < 0.01, data in cultures transfected with hTERT siRNA and treated with HIV-1 in the presence of JSH-23 are significantly different from the corresponding cultures without added JCH-23.
Fig. 5.
HIV-induced alterations of tight junction protein expression in hCMEC/D3 cells are potentiated by hTERT inhibition. hCMEC/D3 cells were transfected or treated with TAG-6 as in Fig. 2. The expression of tight junction proteins, claudin-5 (A and B) and zonula occludens-1 (ZO-1; C and D), were determined by Western blot analysis. The blots are representative images from 3 independent experiments, and the quantified results are depicted in the bar graphs. Results are means ± SE of 3 separate experiments. *P < 0.05 or **P < 0.01, data in cultures exposed to HIV-1 are significantly different from the corresponding controls without HIV treatment. †P < 0.05 or ††P < 0.01, data in cultures with inhibited TERT by hTERT siRNA or TAG-6 and not exposed to HIV are significantly different from the corresponding control cultures without TERT inhibition. ##P < 0.01, data in cultures with inhibited TERT by hTERT siRNA or TAG-6 and treated with HIV-1 are significantly different from the corresponding cultures without TERT inhibition and exposed to HIV-1.
Fig. 6.
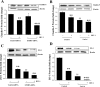
HIV Tat-induced disruption of tight junctions is potentiated in brain microvessels of TERT−/− mice. TERT−/− and WT mice were injected with HIV-1 Tat or vehicle as in Fig. 3. Brain microvessels were isolated 24 h post-Tat injection and analyzed for claudin-5 and ZO-1 expression using immunofluorescence (A and C) and Western blot analysis (B and D) techniques. The immunostaining data (A and C) are merged immunofluorescence and phase-contrast micrographs acquired using a ×60 oil-immersion lens under identical instrument settings. The blots are representative images from 3 mice per group, and the quantified results are depicted in the bar graphs. Results are means ± SE. **P < 0.01, data in WT or TERT−/− mice injected Tat are significantly different from those corresponding controls injected with vehicle. ††P < 0.01, data in TERT−/− mice injected with vehicle are significantly different from the corresponding WT mice injected with vehicle. ##P < 0.01, data in TERT−/− mice treated with Tat are significantly different from the corresponding WT mice injected with Tat.
Fig. 7.
Matrix metalloproteinase-9 (MMP-9) is involved in disruption of claudin-5 expression by HIV-1 and telomerase inhibition. A: hCMEC/D3 cells were transfected with MMP-9 promoter construct (pGL3 MMP-9) or pGL3 MMP-9 with mutated NF-κB binding sites (pGL3 mt-MMP-9). In addition, selected cultures were pretreated with TAG-6 (2 μM) for 2 h and exposed to HIV-1 particles (200 pg p24/ml) for 24 h. B: hCMEC/D3 cells were transfected with control or hTERT siRNA and exposed to HIV-1 as in Fig. 1. MMP-9 activity was assessed by zymography. C: hCMEC/D3 cells were treated as in B. In addition, selected cultures were pretreated for 2 h with GM6001 (general inhibitor of MMP activity; 5 μM) or iMMP-9 (a selective inhibitor of MMP-9, 5 μM). Claudin-5 expression was then determined by Western blot analysis. The blots in B and C are representative images from 3 independent experiments, and the quantified results (means ± SE) are depicted in the bar graphs. *P < 0.05, **P < 0.01, or ***P < 0.001, data in cultures exposed to HIV-1 are significantly different from the corresponding controls without HIV treatment. †P < 0.05, data in cultures with inhibited TERT by hTERT siRNA or TAG-6 and not exposed to HIV are different from the corresponding control cultures without TERT inhibition. ##P < 0.01, data in cultures with inhibited TERT by hTERT siRNA or TAG-6 and treated with HIV-1 are different from the corresponding cultures without TERT inhibition and exposed to HIV-1. ‡‡P < 0.01, data in cultures transfected with hTERT siRNA and treated with HIV-1 in the presence of iMMP-9 or GM6001 are different from the corresponding cultures without added inhibitors.
Similar articles
- HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells.
András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. András IE, et al. J Neurosci Res. 2003 Oct 15;74(2):255-65. doi: 10.1002/jnr.10762. J Neurosci Res. 2003. PMID: 14515355 - PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities.
Huang W, Eum SY, András IE, Hennig B, Toborek M. Huang W, et al. FASEB J. 2009 May;23(5):1596-606. doi: 10.1096/fj.08-121624. Epub 2009 Jan 13. FASEB J. 2009. PMID: 19141539 Free PMC article. - HIV-1 Tat protein alter the tight junction integrity and function of retinal pigment epithelium: an in vitro study.
Bai L, Zhang Z, Zhang H, Li X, Yu Q, Lin H, Yang W. Bai L, et al. BMC Infect Dis. 2008 Jun 6;8:77. doi: 10.1186/1471-2334-8-77. BMC Infect Dis. 2008. PMID: 18538010 Free PMC article. - Telomeres, telomerase, and myc. An update.
Cerni C. Cerni C. Mutat Res. 2000 Jan;462(1):31-47. doi: 10.1016/s1383-5742(99)00091-5. Mutat Res. 2000. PMID: 10648922 Review.
Cited by
- Aging and HIV/AIDS: pathogenetic role of therapeutic side effects.
Torres RA, Lewis W. Torres RA, et al. Lab Invest. 2014 Feb;94(2):120-8. doi: 10.1038/labinvest.2013.142. Epub 2013 Dec 16. Lab Invest. 2014. PMID: 24336070 Free PMC article. Review. - Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability.
Yao H, Duan M, Buch S. Yao H, et al. Blood. 2011 Feb 24;117(8):2538-47. doi: 10.1182/blood-2010-10-313593. Epub 2010 Dec 8. Blood. 2011. PMID: 21148086 Free PMC article. - Delay of endothelial cell senescence protects cerebral barrier against age-related dysfunction: role of senolytics and senomorphics.
Ya J, Kadir RRA, Bayraktutan U. Ya J, et al. Tissue Barriers. 2023 Jul 3;11(3):2103353. doi: 10.1080/21688370.2022.2103353. Epub 2022 Jul 26. Tissue Barriers. 2023. PMID: 35880392 Free PMC article. - Rodent models of HAND and drug abuse: exogenous administration of viral protein(s) and cocaine.
Yao H, Buch S. Yao H, et al. J Neuroimmune Pharmacol. 2012 Jun;7(2):341-51. doi: 10.1007/s11481-012-9355-2. Epub 2012 Mar 24. J Neuroimmune Pharmacol. 2012. PMID: 22447295 Free PMC article. Review. - Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via MAPK signaling.
Wu X, Huang W, Luo G, Alain LA. Wu X, et al. Mol Cell Biochem. 2013 Dec;384(1-2):155-62. doi: 10.1007/s11010-013-1793-5. Epub 2013 Sep 4. Mol Cell Biochem. 2013. PMID: 24002703 Free PMC article.
References
- Ancuta P, Moses A, Gabuzda D. Transendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditions. Immunobiology 209: 11–20, 2004 - PubMed
- Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res 74: 255–265, 2003 - PubMed
- Bartels AL, Kortekaas R, Bart J, Willemsen AT, de Klerk OL, de Vries JJ, van Oostrom JC, Leenders KL. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging 30: 1818–1824, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-39254/NS/NINDS NIH HHS/United States
- R01 MH063022/MH/NIMH NIH HHS/United States
- MH-072567/MH/NIMH NIH HHS/United States
- P42 ES007380/ES/NIEHS NIH HHS/United States
- P20 RR015592/RR/NCRR NIH HHS/United States
- R01 NS039254/NS/NINDS NIH HHS/United States
- MH-63022/MH/NIMH NIH HHS/United States
- P20-RR-15592/RR/NCRR NIH HHS/United States
- R01 MH072567/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases