Perturbation of the P-body component Mov10 inhibits HIV-1 infectivity - PubMed (original) (raw)
Perturbation of the P-body component Mov10 inhibits HIV-1 infectivity
Vyacheslav Furtak et al. PLoS One. 2010.
Abstract
Exogenous retroviruses are obligate cellular parasites that co-opt a number of host proteins and functions to enable their replication and spread. Several host factors that restrict HIV and other retroviral infections have also recently been described. Here we demonstrate that Mov10, a protein associated with P-bodies that has a putative RNA-helicase domain, when overexpressed in cells can inhibit the production of infectious retroviruses. Interestingly, reducing the endogenous Mov10 levels in virus-producing cells through siRNA treatment also modestly suppresses HIV infectivity. The actions of Mov10 are not limited to HIV, however, as ectopic expression of Mov10 restricts the production of other lentiviruses as well as the gammaretrovirus, murine leukemia virus. We found that HIV produced in the presence of high levels of Mov10 is restricted at the pre-reverse transcription stage in target cells. Finally, we show that either helicase mutation or truncation of the C-terminal half of Mov10, where a putative RNA-helicase domain is located, maintained most of its HIV inhibition; whereas removing the N-terminal half of Mov10 completely abolished its activity on HIV. Together these results suggest that Mov10 could be required during the lentiviral lifecycle and that its perturbation disrupts generation of infectious viral particles. Because Mov10 is implicated as part of the P-body complex, these findings point to the potential role of cytoplasmic RNA processing machinery in infectious retroviral production.
Conflict of interest statement
Competing Interests: The corresponding authors Derya Unutmaz and Vineet KewalRamani currently serve as associate editors of PLoS ONE.
Figures
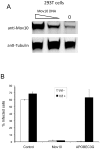
Figure 1. Overexpression of Mov10 decreases HIV-1 infectivity.
(A) 293T cells were transfected with different amounts of Mov10 plasmid, and the expression of Mov10 was determined by Western blot. (B) 293T cells were transfected with 0.5 µg of either pCMV6-XL5 plasmid (control), Mov10 or APOBEC3G in the presence or absence of 0.5 µg vif as well as 1 µg HIV-1-GFP (Δenv, Δvif, Δvpr, Δnef) and 0.5 µg p-L-VSV-G. Virus was collected 24 h later, and then added to Jurkat T cells. Virus transfer was standardized across treatment conditions by p24 levels as described (Materials and Methods). Percent infected cells was then determined using FACS analysis for GFP-expression after virus was allowed to incubate with target cells for 72 hours. Error bars represent one standard deviation.
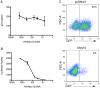
Figure 2. Mov10 decreases specific infectivity of HIV-1.
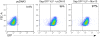
Supernatants of 293T cells that had been transfected with varying amounts of Mov10-expressing plasmid were assayed for (A) HIV-1.Luc p24 levels and (B) after standardization by p24 content, infectivity of target cells was determined by luciferase activity from VSVG.HIV.Luc, which encodes all the HIV accessory genes. For simplicity, the amount of Mov10 plasmid that was transfected is expressed as a ratio of HIV-1 plasmid to Mov10 plasmid and plotted logarithmically. In the experiment (see Materials and Methods for further explanation) HIV-1 plasmid levels remained constant, while Mov10 plasmid was used at levels of 1/6 to 1/1500 that of the HIV-1 plasmid. Error bars represent one standard deviation. (C) Representative plots of Jurkat T cells infected with GFP-expressing virus produced in the presence of either pcDNA3 or Mov10 (ratio of Mov10 to HIV-1 plasmid was 1/25) three days after infection. Quantification of experiments performed using GFP-expressing virus is shown in supplemental figure 1.
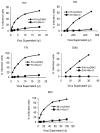
Figure 3. Mov10 impairs HIV-1 replication in primary CD4+ T cells.
Supernatants of activated CD4+ cells that had been nucleofected with a replication competent, CCR5-tropic HIV-1 viral plasmid and either Mov10 or control (pcDNA3) plasmid ([A] schematic) were assayed for p24 production (B) and then standardized by p24 concentration and used to infect CCR5+ Hut cells. (C) Infection success was determined by flow cytometry analysis of GFP expression.
Figure 4. Broad inhibition of infectious retroviruses by Mov10.
Virions derived from 293T cells transfected with various viral plasmids (as described in Materials and Methods) and either pcDNA3 or pcDNA3-Mov10 were used to infect HeLa cells. The % infected cells represents the percentage of GFP-positive cells in the cell population.
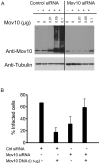
Figure 5. An optimal concentration of Mov10 is required for HIV-1 infectivity.
293T cells were transfected with a non-targeting siRNA or Mov10-specific siRNA. At 48 h post-siRNA transfection, the cells were transfected with 1.5 µg of pHIV-RFP, 0.7 µg of p-L-VSV-G and increasing amounts of the Mov10 expression plasmid. At 96 h post-siRNA transfection: (A) the cell lysates were examined by Western blot for Mov10 levels using an anti-Mov10 antibody. Equal protein loading was confirmed by probing with anti-tubulin antibody. (B) After normalizing for p24 values, virus obtained from the transfections was used to infect HeLa cells and infectivity was measured by FACS. The % infected cells represents the percentage of RFP-positive cells in the cell population. Error bars represent one standard deviation.
Figure 6. Viral glycoprotein incorporation is not affected by Mov10 overexpression.
293T cells were transfected with plasmids necessary for the production of VSV-G pseudotyped Virion-Like Particles (VLPs) containing a GFP tag (as a Gag-GFP fusion) as well as either control or Mov10. Supernatant from these cells was then collected and added to Jurkat T cells and assayed for binding to target cells. Cells bound by one or more VLPs are GFP+ by FACS.
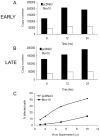
Figure 7. Early and late reverse transcription is suppressed in virus produced from cells overexpressing Mov10.
Synthesis of reverse transcripts by real-time PCR after infection by HIV-1 produced in the presence of either empty vector (pcDNA3) or Mov10 expressing plasmid was measured. No significant difference between production of (A) Early (R-U5) or (B) Late (R-Gag) DNA products of reverse transcription was observed. (C) Infectivity of virions produced in the presence of Mov10 was significantly inhibited. Input viruses were normalized for p24. Results are representative of one out of three similar experiments.
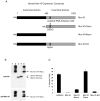
Figure 8. The helicase domain of Mov10 is not required for HIV-1 restriction.
Virions were produced by cotransfection of 293T cells with HIV-1 vector and either empty vector or Mov10, Mov10 N-terminus, Mov10 C-terminus or the putative helicase motif mutant expression plasmids. (A) Schematic of wild-type and mutated human Mov10 constructs. (B) Cell lysates were probed with anti-HA, anti-Mov10, and anti-tubulin. Lanes: 1) pcDNA3, 2) Mov10, 3) Mov10 Nterm, 4) Mov10 Cterm, and 5) Mov10-EQ. Arrows indicate molecular weight of native Mov10. (C) Infectivity of virions produced was examined by FACS after infection of HeLa cells. The % infectivity represents the percentage of RFP-positive cells in the cell population. Error bars represent one standard deviation.
Similar articles
- Moloney leukemia virus 10 (MOV10) protein inhibits retrovirus replication.
Wang X, Han Y, Dang Y, Fu W, Zhou T, Ptak RG, Zheng YH. Wang X, et al. J Biol Chem. 2010 May 7;285(19):14346-55. doi: 10.1074/jbc.M110.109314. Epub 2010 Mar 9. J Biol Chem. 2010. PMID: 20215113 Free PMC article. - P body-associated protein Mov10 inhibits HIV-1 replication at multiple stages.
Burdick R, Smith JL, Chaipan C, Friew Y, Chen J, Venkatachari NJ, Delviks-Frankenberry KA, Hu WS, Pathak VK. Burdick R, et al. J Virol. 2010 Oct;84(19):10241-53. doi: 10.1128/JVI.00585-10. Epub 2010 Jul 28. J Virol. 2010. PMID: 20668078 Free PMC article. - [Host factor Moloney leukemia virus 10 (MOV10) protein inhibits replication of the xenotropic murine leukemia virus-related virus (XMRV)].
Zhang Y, Hu SQ, Pang XJ, Li J, Guo F. Zhang Y, et al. Bing Du Xue Bao. 2014 Sep;30(5):514-20. Bing Du Xue Bao. 2014. PMID: 25562960 Chinese. - Unwinding the roles of RNA helicase MOV10.
Nawaz A, Shilikbay T, Skariah G, Ceman S. Nawaz A, et al. Wiley Interdiscip Rev RNA. 2022 Mar;13(2):e1682. doi: 10.1002/wrna.1682. Epub 2021 Jul 29. Wiley Interdiscip Rev RNA. 2022. PMID: 34327836 Free PMC article. Review. - Cellular RNA helicases and HIV-1: insights from genome-wide, proteomic, and molecular studies.
Chen CY, Liu X, Boris-Lawrie K, Sharma A, Jeang KT. Chen CY, et al. Virus Res. 2013 Feb;171(2):357-65. doi: 10.1016/j.virusres.2012.06.022. Epub 2012 Jul 16. Virus Res. 2013. PMID: 22814432 Free PMC article. Review.
Cited by
- May I Help You with Your Coat? HIV-1 Capsid Uncoating and Reverse Transcription.
Arribas L, Menéndez-Arias L, Betancor G. Arribas L, et al. Int J Mol Sci. 2024 Jun 28;25(13):7167. doi: 10.3390/ijms25137167. Int J Mol Sci. 2024. PMID: 39000271 Free PMC article. Review. - MOV10 recruits DCP2 to decap human LINE-1 RNA by forming large cytoplasmic granules with phase separation properties.
Liu Q, Yi D, Ding J, Mao Y, Wang S, Ma L, Li Q, Wang J, Zhang Y, Zhao J, Guo S, Liu Z, Guo F, Zhao D, Liang C, Li X, Peng X, Cen S. Liu Q, et al. EMBO Rep. 2023 Sep 6;24(9):e56512. doi: 10.15252/embr.202256512. Epub 2023 Jul 12. EMBO Rep. 2023. PMID: 37437058 Free PMC article. - Evolutionary and Expression Analysis of MOV10 and MOV10L1 Reveals Their Origin, Duplication and Divergence.
Yang S, Zhang X, Li X, Yin X, Teng L, Ji G, Li H. Yang S, et al. Int J Mol Sci. 2022 Jul 7;23(14):7523. doi: 10.3390/ijms23147523. Int J Mol Sci. 2022. PMID: 35886872 Free PMC article. - Host Cellular RNA Helicases Regulate SARS-CoV-2 Infection.
Ariumi Y. Ariumi Y. J Virol. 2022 Mar 23;96(6):e0000222. doi: 10.1128/jvi.00002-22. Epub 2022 Feb 2. J Virol. 2022. PMID: 35107372 Free PMC article. - The Interplay Among HIV, LINE-1, and the Interferon Signaling System.
Zhao X, Zhao Y, Du J, Gao P, Zhao K. Zhao X, et al. Front Immunol. 2021 Sep 9;12:732775. doi: 10.3389/fimmu.2021.732775. eCollection 2021. Front Immunol. 2021. PMID: 34566998 Free PMC article. Review.
References
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources