IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism - PubMed (original) (raw)
. 2010 Feb 15;207(2):365-78.
doi: 10.1084/jem.20091777. Epub 2010 Feb 8.
Jonathan M Coquet, Yang Zhang, Amanda Light, Kathy D'Costa, Axel Kallies, Lynn M Corcoran, Dale I Godfrey, Kai-Michael Toellner, Mark J Smyth, Stephen L Nutt, David M Tarlinton
Affiliations
- PMID: 20142430
- PMCID: PMC2822601
- DOI: 10.1084/jem.20091777
IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism
Dimitra Zotos et al. J Exp Med. 2010.
Abstract
Germinal centers (GCs) are sites of B cell proliferation, somatic hypermutation, and selection of variants with improved affinity for antigen. Long-lived memory B cells and plasma cells are also generated in GCs, although how B cell differentiation in GCs is regulated is unclear. IL-21, secreted by T follicular helper cells, is important for adaptive immune responses, although there are conflicting reports on its target cells and mode of action in vivo. We show that the absence of IL-21 signaling profoundly affects the B cell response to protein antigen, reducing splenic and bone marrow plasma cell formation and GC persistence and function, influencing their proliferation, transition into memory B cells, and affinity maturation. Using bone marrow chimeras, we show that these activities are primarily a result of CD3-expressing cells producing IL-21 that acts directly on B cells. Molecularly, IL-21 maintains expression of Bcl-6 in GC B cells. The absence of IL-21 or IL-21 receptor does not abrogate the appearance of T cells in GCs or the appearance of CD4 T cells with a follicular helper phenotype. IL-21 thus controls fate choices of GC B cells directly.
Figures
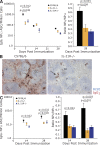
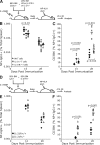
Figure 1.
Loss of IL-21 or IL-21R affects plasma cell formation after TD immunization. (A) The frequency of NP-specific IgG1 ASC among splenocytes from IL-21−/−, IL-21R−/−, and C57BL/6 mice, immunized i.p. with NP-KLH in alum, was assessed by ELISpot at the indicated times after immunization. The results are presented as ASC per million splenocytes (left). At day 28, the ratio of high-affinity (NP2 binding) to total (NP13 binding) IgG1 NP-specific ASC was determined using differentially haptenated plate coats (right). (B) Immunohistochemical staining of spleen sections from C57BL/6 and IL-21R−/− mice at day 7 after immunization. Blue is B220, identifying B cell follicles (F), and red is IgG1, revealing foci of plasma cells (arrows) in C57BL/6 spleens with few in IL-21R−/− spleens. IgG1 staining in follicles marks presumptive immune complexes within GCs. Bars, 100 µm. (C) After immunization, NP-specific IgG1 ASCs appear in the bone marrow of both C57BL/6 and IL-21/IL-21R−/− mice but accumulate only in controls (left), whereas affinity maturation also occurs only in control mice (right) as measured by ELISpot using differentially haptenated plate coats. Results are derived from at least three experiments with 4–10 mice per time point per genotype. Error bars for ELISpot frequency (left) and ratio (right) are SEM. Differences were examined using a Student’s t test and only marked if significant.
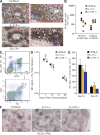
Figure 2.
Intrafollicular response in IL-21– and IL-21R–deficient mice. (A) Representative spleen histology staining revealing the localization of NP-reactive cells (blue) 7 d after immunization in control and IL-21R−/− mice. B cells follicles were stained with IgD (brown). GCs are indicated, as are foci of ASC (open arrows). NP-reactive B cells in follicles are shown by closed arrows in expanded region. Bars, 100 µm. (B) Quantification of the histology staining shown in A from C57BL/6, IL-21−/−, and IL-21R−/− mice of NP-specific plasmablasts (PBs) and plasma cells (PCs) in the red pulp and NP-specific B cell blasts in the follicles. (C) Flow cytometric scheme for the analysis of antigen-specific B cells in the spleens of NP immunized mice. Spleen cells were partitioned into isotype-switched B cells, and the percentage of those cells that were IgG1+ and NP binding was determined to calculate the fraction of total spleen involved in the B cell response. Values shown are the percentage of events displayed and not overall splenic frequencies. (D) Frequency distribution of antigen-reactive B cells in the spleen after immunization of C57BL/6, IL-21−/− and IL-21R−/− mice. (E) Total spleen cell number of antigen-reactive B cells in C57BL/6, IL-21−/−, and IL-21R−/− mice after immunization at the times indicated. Data in D and E are representative of at least three independent experiments of between 5 and 11 mice at each time point. Error bars are ± SEM. Differences were examined using a Student’s t test and only marked if significant. (F) Representative appearance of GC in the spleen by immunohistochemistry, 14 d after immunization, from C57BL/6, IL-21−/−, and IL-21R−/− mice. GC was revealed with PNA (blue). Follicles (F) revealed with B220 (red). Bars, 100 µm.
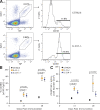
Figure 3.
Accelerated formation of memory B cell compartment in IL-21– and IL-21R–Deficient Mice. (A) Representative flow cytometric plots resolving GC and memory compartments among NP-specific B cells in spleens of C57BL/6 and IL-21R−/− mice 28 d after immunization. Splenocytes, previously gated to include only isotype-switched B cells, were assessed for expression of IgG1 and binding of NP (left). Expression of CD38 on NP+IgG1+ B cells resolved memory (CD38hi) and GC (CD38lo) B cells (right). The percentages are the proportion of displayed events falling within the indicated NP+IgG1+ and CD38hi gates. (B) Proportion of NP-specific B cells with a memory phenotype at times indicated after immunization of C57BL/6, IL-21−/−, and IL-21R−/− mice. (C) Absolute numbers of NP-specific IgG1 memory B cells in spleens of C57BL/6, IL-21−/−, and IL-21R−/− mice at the indicated times after immunization. Symbols in B and C indicate the mean of between 5 and 11 mice at each time point, ± SEM, and derived from at least three independent experiments. Differences were examined using a Student’s t test and only marked if significant.
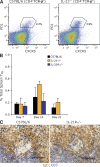
Figure 4.
IL-21 is not essential for Tfh cell development. (A) Representative flow cytometry gating for identifying Tfh cells among splenocytes. CD4+TCR-β+ cells were assessed for expression of PD1 and CXCR5 to resolve Tfh cells in C57BL/6 and IL-21−/− mice. Percentages are of the CD4+TCR-β+ population. (B) Frequency of Tfh cells (CD4+TCR-β+PD1+CXCR5+) in C57BL/6, IL-21−/−, and IL-21R−/− mice at times indicated after immunization. Values are mean frequency of two to six mice per time point from at least two experiments and error bars are ± SD. (C) Immunohistochemical staining of CD3+ cells (blue) in the GCs of the spleens from day 14 immunized C57BL/6 and IL-21R−/− mice. The example shown is representative of at least four mice from two experiments for each genotype. B cell follicles were revealed with IgD (brown). Bars, 100 µm.
Figure 5.
Radiation chimeras show IL-21 acts directly on B cells. (A) Production of chimeric mice with IL-21 or IL-21R deficiency restricted to the T cells. Irradiated recipients were reconstituted with a 1:4 ratio of IL-21R−/− and CD3−/−, IL-21−/− and CD3−/−, or WT and CD3−/− bone marrows and subsequently immunized with NP-KLH in alum. (B) Symbols depict the frequency of NP+IgG1+ B cells in individual chimeric mice at the indicated time in the presence of WT T cells, IL-21−/− T cells, and IL-21R−/− T cells. Mean is indicated by the bar and data are from two independent experiments. (C) The proportion of memory cells among the NP+IgG1+ B populations shown in B. (D) Production of chimeric mice reconstituted with a 1:1 ratio of C57BL/6 (Ly5.1) and IL-21R−/− (Ly5.2) bone marrow cells. (E) Frequency of NP+IgG1+ B cells in individual mice, partitioned according to Ly5 allotype (IL-21R status) at the indicated times with mean shown by the bar. Data are from two independent experiments. (F) The proportion of the NP-specific B cells shown in E with a CD38hi memory phenotype. Significant differences were determined by a Student’s t test and are indicated.
Figure 6.
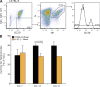
Loss of IL-21 signaling reduces B cell proliferation in GCs. (A) Representative flow cytometric plots for the identification and isolation of NP-specific B cells within the spleens of immunized mice. Spleen cells were enriched for Ig lambda (λ1) expression using magnetic separation then fractionated into isotype switched B cells and the percent of Igλ+ NP binding among those cells determined. Expression of CD38 was used to differentiate germinal GC (CD38lo) from memory B cells (CD38hi) and gates for sorting NP-specific GC B cells set accordingly. (B) Proportion of GC B cells (NP+λ+CD38lo) cells in (S + G2) phase in C57BL/6 and IL-21−/− mice at times indicated. Values are derived from two experiments and are the mean of between three and eight mice (±SD) at each time. Significant difference at day 14 was determined by a Student’s t test. Day-21 data for IL-21–deficient mice are from a pool of three animals because low numbers of GC B cells prevented individual analysis.
Similar articles
- IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses.
Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. Linterman MA, et al. J Exp Med. 2010 Feb 15;207(2):353-63. doi: 10.1084/jem.20091738. Epub 2010 Feb 8. J Exp Med. 2010. PMID: 20142429 Free PMC article. - CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation.
Good-Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. Good-Jacobson KL, et al. J Immunol. 2012 May 1;188(9):4217-25. doi: 10.4049/jimmunol.1102885. Epub 2012 Mar 26. J Immunol. 2012. PMID: 22450810 Free PMC article. - IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation.
Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. Eto D, et al. PLoS One. 2011 Mar 14;6(3):e17739. doi: 10.1371/journal.pone.0017739. PLoS One. 2011. PMID: 21423809 Free PMC article. - System-Level Scenarios for the Elucidation of T Cell-Mediated Germinal Center B Cell Differentiation.
Verstegen NJM, Ubels V, Westerhoff HV, van Ham SM, Barberis M. Verstegen NJM, et al. Front Immunol. 2021 Sep 20;12:734282. doi: 10.3389/fimmu.2021.734282. eCollection 2021. Front Immunol. 2021. PMID: 34616402 Free PMC article. Review. - Development and function of follicular helper T cells.
Ise W. Ise W. Biosci Biotechnol Biochem. 2016;80(1):1-6. doi: 10.1080/09168451.2015.1056512. Epub 2015 Jun 29. Biosci Biotechnol Biochem. 2016. PMID: 26120879 Review.
Cited by
- Cytokine-skewed Tfh cells: functional consequences for B cell help.
Olatunde AC, Hale JS, Lamb TJ. Olatunde AC, et al. Trends Immunol. 2021 Jun;42(6):536-550. doi: 10.1016/j.it.2021.04.006. Epub 2021 May 8. Trends Immunol. 2021. PMID: 33972167 Free PMC article. Review. - IL-21R signaling suppresses IL-17+ gamma delta T cell responses and production of IL-17 related cytokines in the lung at steady state and after Influenza A virus infection.
Moser EK, Sun J, Kim TS, Braciale TJ. Moser EK, et al. PLoS One. 2015 Apr 7;10(4):e0120169. doi: 10.1371/journal.pone.0120169. eCollection 2015. PLoS One. 2015. PMID: 25849970 Free PMC article. - Follicular Helper T (Tfh) Cells in Autoimmune Diseases and Allograft Rejection.
Jeon YH, Choi YS. Jeon YH, et al. Immune Netw. 2016 Aug;16(4):219-32. doi: 10.4110/in.2016.16.4.219. Epub 2016 Aug 23. Immune Netw. 2016. PMID: 27574501 Free PMC article. Review. - Thymic B Cells as a New Player in the Type 1 Diabetes Response.
Greaves RB, Chen D, Green EA. Greaves RB, et al. Front Immunol. 2021 Oct 21;12:772017. doi: 10.3389/fimmu.2021.772017. eCollection 2021. Front Immunol. 2021. PMID: 34745148 Free PMC article. Review. - The role of CD4+ T cells in tumor and chronic viral immune responses.
Xie L, Fang J, Yu J, Zhang W, He Z, Ye L, Wang H. Xie L, et al. MedComm (2020). 2023 Oct 10;4(5):e390. doi: 10.1002/mco2.390. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37829505 Free PMC article. Review.
References
- Cattoretti G., Shaknovich R., Smith P.M., Jäck H.M., Murty V.V., Alobeid B. 2006. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J. Immunol. 177:6930–6939 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous