Genetic approach for intracerebroventricular delivery - PubMed (original) (raw)
Genetic approach for intracerebroventricular delivery
Limor Regev et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Administration of synthetic or purified peptides directly into the brain ventricles is a method commonly used by neuroscientists for exploring physiological and behavioral functions of gene products. i.v. administration is controlled by the blood-brain barrier, which limits its effectiveness, and current approaches for acute or chronic intracerebroventricular delivery have significant technical drawbacks resulting from both the chemical properties of the delivered substance and the experimental procedures. Here we describe a genetic approach for the delivery of secreted peptides or proteins into the cerebrospinal fluid (CSF). Using a choroid plexus-specific promoter, we established a lentiviral-based system, which offers inducible and reversible delivery of a gene product into the CSF. The functionality of this system was demonstrated by using the overexpression of the two established neuropeptides, corticotropin-releasing factor and gonadotropin-releasing hormone, modulating anxiety-like behavior and estrus cycle, respectively. We show that this choroid plexus-specific lentiviral-based system is a reliable, effective, and adaptable research tool for intracerebroventricular delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
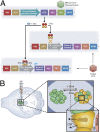
Schematic representation of a lentiviral-based system designed for inducible overexpression of peptides or proteins of interest by the choroid plexus cells. (A) Schematic representation of the Effector (Upper) and Target (Lower) constructs. (B) ICV injection of the Effector and Target viruses (Left) results in a Dox inducible transcription of the transgene by the choroid plexus cells (Large Inset) and delivery of the gene product into the CSF (Small Inset). rtTA, reverse tetracycline trans activator; TRE, tetracycline-responsive element; ISF, interstitial fluid.
Fig. 2.
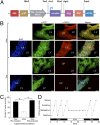
Choroid plexus specificity and in vivo validation of constructed lentiviruses. (A) Schematic representation of the choroid plexus-specific Effector lentiviral construct, containing 2.5 kb of the 5′-flanking region of the CRFR2β gene, which drives the transcription of the rtTA and the GFP proteins. (B) Semiquantitative RT-PCR analysis, using specific primers for mouse CRFR2β, demonstrates abundant expression of CRFR2β in the choroid plexus but not in hypothalamic tissue (HT). (C) Dark-field photomicrographs showing positive hybridization signal for mouse CRFR2β in the choroid plexus. (D) ICV injection of the choroid plexus-specific Effector lentiviruses resulted in GFP expression specifically by the choroid plexus and not the ependymal cells. Higher magnification images demonstrate the ability of these lentiviruses to infect the choroid plexus cells with high efficiency. (Scale bars: Upper panels 0.2 mm; lower panels 0.1 mm.) CP, choroid plexus; LV, lateral ventricle.
Fig. 3.
Precursors processing and posttranscriptional modifications by the choroid plexus tissue. Semiquantitative RT-PCR analysis of the cleaving enzyme Furin (A), PC1 (B), PC2 (C), CPE (D), PAM1 (E), and the ribosomal protein S16 (F), performed on cDNA isolated from the mouse choroid plexus (CP) and hypothalamic tissue (HT). (G) Schematic representation of the Target lentiviral construct, designed to conditionally express additional enzyme or cofactor, followed by a blue florescent protein (BFP). (H) Infection of HEK-293T cells with the three reporter lentiviruses showed the feasibility of a triple infection, as can be seen in the fluorescent visualization. (Scale bars: 0.15 mm.)
Fig. 4.
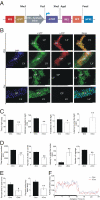
Inducible overexpression of mCRF in the CSF using the choroid plexus-specific lentiviral-based system and the subsequent anxiogenic behavior. (A) Schematic representation of the CRF-Target lentiviral construct, designed to conditionally express mCRF, followed by a RFP. (B) Immunohistochemical analysis of brain slices obtained from mice injected with a mixture of the Effector and CRF-Target lentiviruses and kept under induced (+Dox) or noninduced (-Dox) conditions show a choroid plexus-specific staining for GFP under both induced and noninduced conditions (green fluorescence) and a specific Dox-dependent staining for mouse CRF (red fluorescence). (Scale bars: long: 0.1 mm; short: 0.15 mm.) (C) Mice injected with a mixture of the Effector and CRF-Target lentiviruses and kept under induced (+Dox) conditions for 3 days, showed a significant increase in anxiety-like behavior measured by the light/dark transfer test, the open field test (D) and the elevated plus maze test (E). (F) No significant differences were found between the experimental groups in their home cage locomotor activity. Values are expressed as mean ± SEM. **, P < 0.005; *, P < 0.05. n = 10–12 male C57BL/6 mice were used in each of the behavioral tests.
Fig. 5.
Inducible overexpression of mGnRH in the CSF using a choroid plexus-specific lentiviral based system and the subsequent estrus cycle modulation. (A) Schematic representation of the GnRH-Target lentiviral construct, designed to conditionally express mGnRH, followed by a RFP. (B) Immunohistochemical analysis of brain slices obtained from mice injected with a mixture of the Effector and GnRH-Target lentiviruses and kept under induced (+Dox) or noninduced (-Dox) conditions show a choroid plexus-specific staining for GFP under both induced and noninduced conditions (green fluorescence) and a specific Dox-dependent staining for mouse GnRH (red fluorescence). (Scale bars: long: 0.1 mm; short: 0.15 mm) (C) The estrus cycle length of female mice injected with a mixture of the Effector and GnRH-Target lentiviruses was determined prior, during, and after Dox administration. Estrous cycle determination showed that while most of the injected animals show a normal 4-day estrous cycle under noninduced conditions, Dox administration significantly disrupted the cycle integrity. After removal of Dox from drinking water most of the mice reestablished an intact 4-day cycle (D) A representative estrous cycle profile of a female mouse throughout the experiment. Values are expressed as mean ± SEM. *, P < 0.0001. n = 30 female ICR mice were used for the estrous cycle studies.
Similar articles
- Development of BMP7-producing human cells, using a third generation lentiviral gene delivery system.
Chitty DW, Tremblay RG, Ribecco-Lutkiewicz M, Haukenfrers J, Zurakowski B, Massie B, Sikorska M, Bani-Yaghoub M. Chitty DW, et al. J Neurosci Methods. 2012 Mar 30;205(1):17-27. doi: 10.1016/j.jneumeth.2011.12.007. Epub 2011 Dec 22. J Neurosci Methods. 2012. PMID: 22209770 - Different Serotypes of Adeno-Associated Virus Vector- and Lentivirus-Mediated Tropism in Choroid Plexus by Intracerebroventricular Delivery.
Chen X, He Y, Tian Y, Wang Y, Wu Z, Lan T, Wang H, Cheng K, Xie P. Chen X, et al. Hum Gene Ther. 2020 Apr;31(7-8):440-447. doi: 10.1089/hum.2019.300. Epub 2020 Mar 4. Hum Gene Ther. 2020. PMID: 32056463 - Correction of murine mucopolysaccharidosis type IIIA central nervous system pathology by intracerebroventricular lentiviral-mediated gene delivery.
McIntyre C, Derrick-Roberts AL, Byers S, Anson DS. McIntyre C, et al. J Gene Med. 2014 Nov-Dec;16(11-12):374-87. doi: 10.1002/jgm.2816. J Gene Med. 2014. PMID: 25418946 - Experimental design for stable genetic manipulation in mammalian cell lines: lentivirus and alternatives.
Shearer RF, Saunders DN. Shearer RF, et al. Genes Cells. 2015 Jan;20(1):1-10. doi: 10.1111/gtc.12183. Epub 2014 Oct 13. Genes Cells. 2015. PMID: 25307957 Review.
Cited by
- The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response.
Dedic N, Chen A, Deussing JM. Dedic N, et al. Curr Mol Pharmacol. 2018;11(1):4-31. doi: 10.2174/1874467210666170302104053. Curr Mol Pharmacol. 2018. PMID: 28260504 Free PMC article. Review. - AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer's Disease.
Rosenberg JB, Kaplitt MG, De BP, Chen A, Flagiello T, Salami C, Pey E, Zhao L, Ricart Arbona RJ, Monette S, Dyke JP, Ballon DJ, Kaminsky SM, Sondhi D, Petsko GA, Paul SM, Crystal RG. Rosenberg JB, et al. Hum Gene Ther Clin Dev. 2018 Mar;29(1):24-47. doi: 10.1089/humc.2017.231. Epub 2018 Mar 13. Hum Gene Ther Clin Dev. 2018. PMID: 29409358 Free PMC article. - Choroid Plexus: The Orchestrator of Long-Range Signalling Within the CNS.
Kaiser K, Bryja V. Kaiser K, et al. Int J Mol Sci. 2020 Jul 4;21(13):4760. doi: 10.3390/ijms21134760. Int J Mol Sci. 2020. PMID: 32635478 Free PMC article. Review. - Experimental approaches for manipulating choroid plexus epithelial cells.
Jang A, Lehtinen MK. Jang A, et al. Fluids Barriers CNS. 2022 May 26;19(1):36. doi: 10.1186/s12987-022-00330-2. Fluids Barriers CNS. 2022. PMID: 35619113 Free PMC article. Review. - The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond.
Zappaterra MW, Lehtinen MK. Zappaterra MW, et al. Cell Mol Life Sci. 2012 Sep;69(17):2863-78. doi: 10.1007/s00018-012-0957-x. Epub 2012 Mar 14. Cell Mol Life Sci. 2012. PMID: 22415326 Free PMC article. Review.
References
- Saunders NR, Ek CJ, Habgood MD, Dziegielewska KM. Barriers in the brain: A renaissance? Trends Neurosci. 2008;31:279–286. - PubMed
- Pardridge WM. Drug and gene delivery to the brain: The vascular route. Neuron. 2002;36:555–558. - PubMed
- Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. - PubMed
- Johanson CE, Duncan JA, Stopa EG, Baird A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res. 2005;22:1011–1037. - PubMed
- Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. Bioessays. 2005;27:262–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources