Biomaterials/tissue interactions: possible solutions to overcome foreign body response - PubMed (original) (raw)
Review
Biomaterials/tissue interactions: possible solutions to overcome foreign body response
Jacqueline M Morais et al. AAPS J. 2010 Jun.
Abstract
In recent years, a variety of biomaterial implantable devices has been developed. Of particular significance to pharmaceutical sciences is the progress made on the development of drug/implantable device combination products. However, the clinical application of these devices is still a critical issue due to the host response, which results from both the tissue trauma during implantation and the presence of the device in the body. Accordingly, the in vivo functionality and durability of any implantable device can be compromised by the body response to the foreign material. Numerous strategies to overcome negative body reactions have been reported. The aim of this review is to outline some key issues of biomaterial/tissue interactions such as foreign body response and biocompatibility and biocompatibility assessment. In addition, general approaches used to overcome the in vivo instability of implantable devices are presented, including (a) biocompatible material coatings, (b) steroidal and nonsteroidal anti-inflammatory drugs, and (c) angiogenic drugs. In particular, strategies to overcome host response to glucose biosensors are summarized.
Figures
Fig. 1
Sequence of events involved in the FBR to an implantable device. Note that overlap and simultaneous occurrence of these events occurs (based on (21,33))
Fig. 2
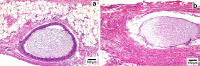
Pharmacodynamic changes in representative tissue sections on day 3 from s.c. tissue of rats implanted with PLGA microsphere/PVA hydrogel composites: a without dexamethasone and b with dexamethasone compared with control untreated tissue sections (c). Inflammation-mediating cells and normal cells are stained purple and pink, respectively (hematoxylin and eosin stain). Hydrogel composites are marked HC
Fig. 3
Pharmacodynamic changes in representative tissue sections on day 21 from s.c. tissue of rats implanted with PLGA microsphere/PVA hydrogel composites: a without dexamethasone and b with dexamethasone. Inflammation-mediating cells and normal cells are stained purple and pink, respectively (hematoxylin and eosin stain). The white area surrounding the hydrogel composite in b is an artifact due to tissue detachment during sectioning. Hydrogel composites are marked HC
Fig. 4
Pharmacodynamics changes in representative subcutaneous tissue sections of rats implanted with PLGA microsphere/PVA hydrogel composites (asterisk) containing VEGF alone over week 1 (a), week 2 (b), week 3 (c), and week 4 (d) postimplantation and dexamethasone and VEGF combination week 1 (e); week 2 (f); week 3 (g), and week postimplantation (h). Inflammation-mediating cells and normal cells are stained purple and pink, respectively (hematoxylin and eosin stain)
Similar articles
- Polymeric "smart" coatings to prevent foreign body response to implantable biosensors.
Wang Y, Papadimitrakopoulos F, Burgess DJ. Wang Y, et al. J Control Release. 2013 Aug 10;169(3):341-7. doi: 10.1016/j.jconrel.2012.12.028. Epub 2013 Jan 5. J Control Release. 2013. PMID: 23298616 - Glucose sensor membranes for mitigating the foreign body response.
Koh A, Nichols SP, Schoenfisch MH. Koh A, et al. J Diabetes Sci Technol. 2011 Sep 1;5(5):1052-9. doi: 10.1177/193229681100500505. J Diabetes Sci Technol. 2011. PMID: 22027297 Free PMC article. - Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants.
Kastellorizios M, Papadimitrakopoulos F, Burgess DJ. Kastellorizios M, et al. J Control Release. 2015 Sep 28;214:103-11. doi: 10.1016/j.jconrel.2015.07.021. Epub 2015 Jul 26. J Control Release. 2015. PMID: 26216396 - The foreign body response: at the interface of surgery and bioengineering.
Major MR, Wong VW, Nelson ER, Longaker MT, Gurtner GC. Major MR, et al. Plast Reconstr Surg. 2015 May;135(5):1489-1498. doi: 10.1097/PRS.0000000000001193. Plast Reconstr Surg. 2015. PMID: 25919260 Review. - Materials Strategies to Overcome the Foreign Body Response.
Zhou X, Wang Y, Ji J, Zhang P. Zhou X, et al. Adv Healthc Mater. 2024 Jul;13(18):e2304478. doi: 10.1002/adhm.202304478. Epub 2024 May 2. Adv Healthc Mater. 2024. PMID: 38666550 Review.
Cited by
- Modulation of Inflammatory Response and Induction of Bone Formation Based on Combinatorial Effects of Resveratrol.
Rutledge KE, Cheng Q, Jabbarzadeh E. Rutledge KE, et al. J Nanomed Nanotechnol. 2016 Feb;7(1):350. doi: 10.4172/2157-7439.1000350. Epub 2016 Jan 25. J Nanomed Nanotechnol. 2016. PMID: 27175310 Free PMC article. - Live imaging the foreign body response in zebrafish reveals how dampening inflammation reduces fibrosis.
Gurevich DB, French KE, Collin JD, Cross SJ, Martin P. Gurevich DB, et al. J Cell Sci. 2019 Sep 26;133(5):jcs236075. doi: 10.1242/jcs.236075. J Cell Sci. 2019. PMID: 31444283 Free PMC article. - Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings.
Shen J, Burgess DJ. Shen J, et al. Int J Pharm. 2012 Jan 17;422(1-2):341-8. doi: 10.1016/j.ijpharm.2011.10.020. Epub 2011 Oct 13. Int J Pharm. 2012. PMID: 22016033 Free PMC article. - Biodegradable polymerized simvastatin stimulates bone formation.
Venkatesan N, Liyanage ADT, Castro-Núñez J, Asafo-Adjei T, Cunningham LL, Dziubla TD, Puleo DA. Venkatesan N, et al. Acta Biomater. 2019 Jul 15;93:192-199. doi: 10.1016/j.actbio.2019.04.059. Epub 2019 May 2. Acta Biomater. 2019. PMID: 31055123 Free PMC article. - Design and fabrication of a high-performance electrochemical glucose sensor.
Vaddiraju S, Legassey A, Wang Y, Qiang L, Burgess DJ, Jain F, Papadimitrakopoulos F. Vaddiraju S, et al. J Diabetes Sci Technol. 2011 Sep 1;5(5):1044-51. doi: 10.1177/193229681100500504. J Diabetes Sci Technol. 2011. PMID: 22027296 Free PMC article.
References
- Prokop A. Bioartificial organs in the twenty-first century: nano-biological devices. Ann NY Acad Sci. 2001;944:472–490. - PubMed
- Wilson GS, Zhang Y, Reach G, Moatti-Sirat D, Poitout V, Thévenot DR, et al. Progress toward the development of an implantable sensor for glucose. Clin Chem. 1992;38(9):1613–1617. - PubMed
- Klonoff DC. Technological advances in the treatment of diabetes mellitus: better bioengineering begets benefits in glucose measurement, the artificial pancreas, and insulin delivery. Pediatr Endocrinol Rev. 2003;1(2):94–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources