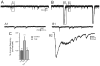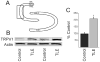Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy - PubMed (original) (raw)
Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy
Muthu D Bhaskaran et al. Exp Neurol. 2010 Jun.
Abstract
Temporal lobe epilepsy (TLE) is a condition characterized by an imbalance between excitation and inhibition in the temporal lobe. Hallmarks of this change are axon sprouting and accompanying synaptic reorganization in the temporal lobe. Synthetic and endogenous cannabinoids have variable therapeutic potential in treating intractable temporal lobe epilepsy, in part because cannabinoid ligands can bind multiple receptor types. This study utilized in vitro electrophysiological methods to examine the effect of transient receptor potential vanilloid type 1 (TRPV1) activation in dentate gyrus granule cells in a murine model of TLE. Capsaicin, a selective TRPV1 agonist had no measurable effect on overall synaptic input to granule cells in control animals, but significantly enhanced spontaneous and miniature EPSC frequency in mice with TLE. Exogenous application of anandamide, an endogenous cannabinoid that acts at both TRPV1 and cannabinoid type 1 receptors (CB1R), also enhanced glutamate release in the presence of a CB1R antagonist. Anandamide reduced the EPSC frequency when TRPV1 were blocked with capsazepine. Western blot analysis of TRPV1 receptor indicated protein expression was significantly greater in the dentate gyrus of mice with TLE compared with control mice. This study indicates that a prominent cannabinoid agonist can increase excitatory circuit activity in the synaptically reorganized dentate gyrus of mice with TLE by activating TRPV1 receptors, and suggests caution in designing anticonvulsant therapy based on modulating the endocannabinoid system.
Copyright (c) 2009 Elsevier Inc. All rights reserved.
Figures
Figure 1
Timm staining showing mossy fiber sprouting into the inner molecular layer of the dentate gyrus in pilocarpine-treated mice. A. Dentate gyrus of a normal mouse. B. Dentate gyrus of a pilocarpine-treated mouse that survived SE showing mossy fiber sprouting. A1 and B1 are enlarged boxed regions of A and B. The arrows in B and B1 point to extensive mossy fiber sprouting in the inner molecular layer of the dentate gyrus.
Figure 2
Effect of capsaicin (Cap) on sEPSCs recorded from pilocarpine-treated mice with TLE. A. control trace recorded from a granule cell of a pilocarpine-treated mouse that survived SE. B. Addition of capsaicin increased the frequency of sEPSCs. A1, B1 and B are 2 expanded regions of the boxed areas of A and B. C. Normalized graph showing cap did not have effect on sEPSCs of control mice (p<0.05). In mice with TLE, cap significantly increased sEPSC frequency (asterisk indicates p<0.05) and this effect was blocked by pre-application of capsazepine (CPZ). Number of neurons is indicated in parentheses for each condition.
Figure 3
Capsaicin increased frequency of mEPSCs in pilocarpine-treated mice. A. mEPSCs recorded from granule cell of a pilocarpine-treated mouse in the presence of TTX (1μM). B. mEPSC frequency increased with the application of capsaicin. A1 and B1 are expanded regions of A and B. C. Kolmogrov-Smirnov analysis showing reduction of inter-event interval after application of capsaicin in the same recording. D. Cumulative normalized data from control mice and pilocarpine-treated mice showing that capsaicin increased frequency of mEPSCs in pilocarpine-treated mice but not in controls. * indicates significance (P<0.05) versus control conditions. Number of neurons is indicated in parentheses for each condition.
Figure 4
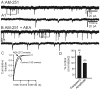
AEA increased the frequency of mEPSCs in the presence of CB1R antagonist, AM 251. A. Control trace recorded from a granule cell of a pilocarpine-treated mouse showing mEPSCs in the presence of AM 251. B. Trace showing increase in frequency after addition of AEA. A1 and B1 are expanded segments indicated by the boxed areas in A and B respectively. C. Kolmogrov-Smirnov analysis of the same recording showing reduction in the inter-event interval after the addition of AEA. D. Cumulative normalized data showing an increase in mEPSC frequency induced by AEA when CB1R were blocked in neurons from seven pilocarpine-treated mice with TLE. * indicates significant change (p<0.05) versus control conditions. Number of neurons is indicated in parentheses for each condition.
Figure 5
AEA decreased the frequency of mEPSCs in the presence of the TRPV1 receptor antagonist, CPZ. A. Control trace recorded from a granule cell of a pilocarpine-treated mouse showing mEPSCs in the presence of CPZ. B. Trace illustrating a decrease in the frequency of mEPSCs after the addition of AEA. A1 and B1 are expanded segments indicated by the boxed areas in A and B respectively. C. Kolmogrov-Smirnov analysis showing an increase in the inter-event interval after the addition of AEA. D. Cumulative normalized data indicating a decrease in mEPSC frequency induced by AEA when TRPV1 receptors were blocked in neurons from six mice with TLE. * indicates significant change (p<0.05) versus control conditions. Number of neurons is indicated in parentheses for each condition.
Figure 6
Western blot detection of TRPV1 receptor expression in the dentate gyrus. A. Diagram of dentate gyrus showing the micro-dissected area (box). B. Western blot showing TRPV1 receptor expression in two untreated mice and in two pilocarpine-treated mice that survived SE. Actin was used as the loading control which did not change significantly. C. Graph showing significant (p<0.05; n=4) increase in TRPV1 receptor expression in epileptic mice.
Similar articles
- Cannabinoid-mediated inhibition of recurrent excitatory circuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy.
Bhaskaran MD, Smith BN. Bhaskaran MD, et al. PLoS One. 2010 May 17;5(5):e10683. doi: 10.1371/journal.pone.0010683. PLoS One. 2010. PMID: 20498848 Free PMC article. - Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey.
Kawahara H, Drew GM, Christie MJ, Vaughan CW. Kawahara H, et al. Br J Pharmacol. 2011 Jul;163(6):1214-22. doi: 10.1111/j.1476-5381.2010.01157.x. Br J Pharmacol. 2011. PMID: 21175570 Free PMC article. - Involvement of TRPV1 channels in the activity of the cannabinoid WIN 55,212-2 in an acute rat model of temporal lobe epilepsy.
Carletti F, Gambino G, Rizzo V, Ferraro G, Sardo P. Carletti F, et al. Epilepsy Res. 2016 May;122:56-65. doi: 10.1016/j.eplepsyres.2016.02.005. Epilepsy Res. 2016. PMID: 26970948 - Why do cannabinoid receptors have more than one endogenous ligand?
Di Marzo V, De Petrocellis L. Di Marzo V, et al. Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3216-28. doi: 10.1098/rstb.2011.0382. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108541 Free PMC article. Review. - Roles of transient receptor potential vanilloid subtype 1 and cannabinoid type 1 receptors in the brain: neuroprotection versus neurotoxicity.
Kim SR, Chung YC, Chung ES, Park KW, Won SY, Bok E, Park ES, Jin BK. Kim SR, et al. Mol Neurobiol. 2007 Jun;35(3):245-54. doi: 10.1007/s12035-007-0030-1. Mol Neurobiol. 2007. PMID: 17917113 Review.
Cited by
- Kainic Acid Activates TRPV1 via a Phospholipase C/PIP2-Dependent Mechanism in Vitro.
Mohandass A, Surenkhuu B, Covington K, Baskaran P, Lehmann T, Thyagarajan B. Mohandass A, et al. ACS Chem Neurosci. 2020 Oct 7;11(19):2999-3007. doi: 10.1021/acschemneuro.0c00297. Epub 2020 Sep 11. ACS Chem Neurosci. 2020. PMID: 32833423 Free PMC article. - α-Spinasterol, a TRPV1 receptor antagonist, elevates the seizure threshold in three acute seizure tests in mice.
Socała K, Nieoczym D, Pieróg M, Wlaź P. Socała K, et al. J Neural Transm (Vienna). 2015 Sep;122(9):1239-47. doi: 10.1007/s00702-015-1391-7. Epub 2015 Mar 13. J Neural Transm (Vienna). 2015. PMID: 25764210 Free PMC article. - Neural Stem Cells and Cannabinoids in the Spotlight as Potential Therapy for Epilepsy.
Lourenço DM, Ribeiro-Rodrigues L, Sebastião AM, Diógenes MJ, Xapelli S. Lourenço DM, et al. Int J Mol Sci. 2020 Oct 3;21(19):7309. doi: 10.3390/ijms21197309. Int J Mol Sci. 2020. PMID: 33022963 Free PMC article. Review. - Toll-like receptor 4 enhancement of non-NMDA synaptic currents increases dentate excitability after brain injury.
Li Y, Korgaonkar AA, Swietek B, Wang J, Elgammal FS, Elkabes S, Santhakumar V. Li Y, et al. Neurobiol Dis. 2015 Feb;74:240-53. doi: 10.1016/j.nbd.2014.11.021. Epub 2014 Dec 8. Neurobiol Dis. 2015. PMID: 25497689 Free PMC article. - Complement C3 Aggravates Post-epileptic Neuronal Injury Via Activation of TRPV1.
Jiang GT, Shao L, Kong S, Zeng ML, Cheng JJ, Chen TX, Han S, Yin J, Liu WH, He XH, Liu YM, Gongga L, Peng BW. Jiang GT, et al. Neurosci Bull. 2021 Oct;37(10):1427-1440. doi: 10.1007/s12264-021-00750-4. Epub 2021 Jul 26. Neurosci Bull. 2021. PMID: 34309810 Free PMC article.
References
- Al-Hayani A, Wease KN, Ross RA, Pertwee RG, Davies SN. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41:1000–1005. - PubMed
- Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18:333–340. - PubMed
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. - PubMed
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. - PubMed
- Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources