Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization - PubMed (original) (raw)
Comparative Study
Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization
Anita Hill et al. Haematologica. 2010 Apr.
Abstract
Background: Paroxysmal nocturnal hemoglobinuria is an acquired hemolytic anemia characterized by intravascular hemolysis which has been demonstrated to be effectively controlled with eculizumab. However, lactate dehydrogenase levels remain slightly elevated and haptoglobin levels remain low in some patients suggesting residual low-level hemolysis. This may be due to C3-mediated clearance of paroxysmal nocturnal hemoglobinuria red blood cells through the reticuloendothelial system.
Design and methods: Thirty-nine samples from patients not treated with eculizumab and 31 samples from patients treated with eculizumab were obtained (for 17 of these 31 samples there were also samples taken prior to eculizumab treatment). Membrane bound complement was assessed by flow cytometry. Direct antiglobulin testing was carried out using two methods. Lactate dehydrogenase was assayed to assess the degree of hemolysis.
Results: Three of 39 patients (8%) with paroxysmal nocturnal hemoglobinuria not on eculizumab had a positive direct antiglobulin test, while the test was positive in 21 of 31 (68%) during eculizumab treatment. Of these 21 patients who had a positive direct antiglobulin test during eculizumab treatment, 17 had been tested prior to treatment; only one was positive. Flow cytometry using anti-C3 monoclonal antibodies was performed on the 21 direct antiglobulin test-positive, eculizumab-treated patients; the median proportion of C3-positive total red blood cells was 26%. Among the eculizumab-treated patients, 16 of the 21 (76.2%) with a positive direct antiglobulin test received at least one transfusion compared with one of ten (10.0%) of those with a negative test (P<0.01). Among the eculizumab-treated patients, the mean hemoglobin value for the 21 with a positive direct antiglobulin test was 9.6+/-0.3 g/dL, whereas that in the ten patients with a negative test was 11.0+/-0.4 g/dL (P=0.02).
Conclusions: These data demonstrate a previously masked mechanism of red cell clearance in paroxysmal nocturnal hemoglobinuria and suggests that blockade of complement at C5 allows C3 fragment accumulation on some paroxysmal nocturnal hemoglobinuria red cells, explaining the residual low-level hemolysis occurring in some eculizumab-treated patients.
Figures
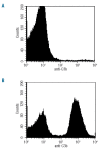
Figure 1.
Surface levels of C3b fragment determined by flow cytometry. A fluorescent anti-C3b antibody was used against (A) washed whole blood from normal volunteers (negative control) and (B) washed whole blood from normal volunteers incubated with neat serum from a patient with cold hemagglutinin disease and neat C8d serum (positive control) (x axis = fluorescent intensity; log scale).
Figure 2.
Direct antiglobulin test (DAT) in patients with PNH in the presence or absence of the complement inhibitor eculizumab. Proportions of DAT-positive individuals among normal volunteers, PNH patients not receiving eculizumab, and PNH patients receiving eculizumab are shown.
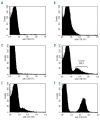
Figure 3.
Flow cytometric analysis of C3b fragment deposition on red cells from PNH patients before or during eculizumab treatment. Levels of C3b on A) red cells from a normal volunteer; B) and C) red cells from a PNH patient not on eculizumab; D), E) and F) red cells from PNH patients on eculizumab.
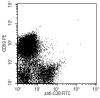
Figure 4.
Two-color flow cytometric analysis of C3b fragment deposition on red cells from PNH patients during eculizumab treatment. A proportion of CD59-negative red cells are C3b-positive whereas the non-PNH (CD59-positive red cells) remain negative for C3b.
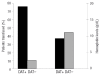
Figure 5.
Transfusion requirement and hemoglobin values in DAT-positive or -negative PNH patients treated with eculizumab. Proportion of patients who received at least one transfusion and the level of hemoglobin in DAT-positive (DAT +; n=21) and DAT-negative (DAT -; n=10) PNH patients on eculizumab therapy.
Comment in
- Paroxysmal nocturnal hemoglobinuria and eculizumab.
Luzzatto L, Risitano AM, Notaro R. Luzzatto L, et al. Haematologica. 2010 Apr;95(4):523-6. doi: 10.3324/haematol.2009.017848. Haematologica. 2010. PMID: 20378572 Free PMC article. No abstract available.
Similar articles
- Extravascular hemolysis and complement consumption in Paroxysmal Nocturnal Hemoglobinuria patients undergoing eculizumab treatment.
Subías Hidalgo M, Martin Merinero H, López A, Anter J, García SP, Ataúlfo Gonzalez-Fernández F, Forés R, Lopez-Trascasa M, Villegas A, Ojeda E, Rodríguez de Córdoba S. Subías Hidalgo M, et al. Immunobiology. 2017 Feb;222(2):363-371. doi: 10.1016/j.imbio.2016.09.002. Epub 2016 Sep 13. Immunobiology. 2017. PMID: 27644115 - Anti-complement Treatment for Paroxysmal Nocturnal Hemoglobinuria: Time for Proximal Complement Inhibition? A Position Paper From the SAAWP of the EBMT.
Risitano AM, Marotta S, Ricci P, Marano L, Frieri C, Cacace F, Sica M, Kulasekararaj A, Calado RT, Scheinberg P, Notaro R, Peffault de Latour R. Risitano AM, et al. Front Immunol. 2019 Jun 14;10:1157. doi: 10.3389/fimmu.2019.01157. eCollection 2019. Front Immunol. 2019. PMID: 31258525 Free PMC article. Review. - Eculizumab treatment: stochastic occurrence of C3 binding to individual PNH erythrocytes.
Sica M, Rondelli T, Ricci P, De Angioletti M, Risitano AM, Notaro R. Sica M, et al. J Hematol Oncol. 2017 Jun 19;10(1):126. doi: 10.1186/s13045-017-0496-x. J Hematol Oncol. 2017. PMID: 28629435 Free PMC article. - C3-mediated extravascular hemolysis in PNH on eculizumab: Mechanism and clinical implications.
Notaro R, Sica M. Notaro R, et al. Semin Hematol. 2018 Jul;55(3):130-135. doi: 10.1053/j.seminhematol.2018.05.014. Epub 2018 Jun 5. Semin Hematol. 2018. PMID: 30032749 Review.
Cited by
- SAR443809: a selective inhibitor of the complement alternative pathway, targeting complement factor Bb.
Rajagopal V, Leksa N, Gorham R, Jindal S, Nair S, Knockenhauer K, Chan J, Byun T, Mercadante C, Moore S, Panicker S, Parry G, Storek M. Rajagopal V, et al. Blood Adv. 2023 Aug 22;7(16):4258-4268. doi: 10.1182/bloodadvances.2022009028. Blood Adv. 2023. PMID: 36897252 Free PMC article. - Eculizumab: a review of its use in paroxysmal nocturnal haemoglobinuria.
McKeage K. McKeage K. Drugs. 2011 Dec 3;71(17):2327-45. doi: 10.2165/11208300-000000000-00000. Drugs. 2011. PMID: 22085388 Review. - Role of Complement in Autoimmune Hemolytic Anemia.
Berentsen S. Berentsen S. Transfus Med Hemother. 2015 Sep;42(5):303-10. doi: 10.1159/000438964. Epub 2015 Sep 7. Transfus Med Hemother. 2015. PMID: 26696798 Free PMC article. Review. - Phase 2 study of danicopan in patients with paroxysmal nocturnal hemoglobinuria with an inadequate response to eculizumab.
Kulasekararaj AG, Risitano AM, Maciejewski JP, Notaro R, Browett P, Lee JW, Huang M, Geffner M, Brodsky RA. Kulasekararaj AG, et al. Blood. 2021 Nov 18;138(20):1928-1938. doi: 10.1182/blood.2021011388. Blood. 2021. PMID: 34314483 Free PMC article. Clinical Trial. - Complement C3 inhibition by compstatin Cp40 prevents intra- and extravascular hemolysis of red blood cells.
Baas I, Delvasto-Nuñez L, Ligthart P, Brouwer C, Folman C, Reis ES, Ricklin D, Lambris JD, Wouters D, de Haas M, Jongerius I, Zeerleder SS. Baas I, et al. Haematologica. 2020 Jan 31;105(2):e57-e60. doi: 10.3324/haematol.2019.216028. Print 2020. Haematologica. 2020. PMID: 31171642 Free PMC article. No abstract available.
References
- Hill A, Richards SJ, Hillmen P. Recent developments in the understanding and management of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2007;137(3):181–92. - PubMed
- Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253–8. - PubMed
- Clark DA, Butler SA, Braren V, Hartmann RC, Jenkins DE., Jr The kidneys in paroxysmal nocturnal hemoglobinuria. Blood. 1981;57(1):83–9. - PubMed
- Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33(17–18):1389–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous