SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions - PubMed (original) (raw)
Review
SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions
Cynthia Detre et al. Semin Immunopathol. 2010 Jun.
Abstract
One or more of the signaling lymphocytic activation molecule (SLAM) family (SLAMF) of cell surface receptors, which consists of nine transmembrane proteins, i.e., SLAMF1-9, are expressed on most hematopoietic cells. While most SLAMF receptors serve as self-ligands, SLAMF2 and SLAMF4 use each other as counter structures. Six of the receptors carry one or more copies of a unique intracellular tyrosine-based switch motif, which has high affinity for the single SH2-domain signaling molecules SLAM-associated protein and EAT-2. Whereas SLAMF receptors are costimulatory molecules on the surface of CD4+, CD8+, and natural killer (NK) T cells, they also involved in early phases of lineage commitment during hematopoiesis. SLAMF receptors regulate T lymphocyte development and function and modulate lytic activity, cytokine production, and major histocompatibility complex-independent cell inhibition of NK cells. Furthermore, they modulate B cell activation and memory generation, neutrophil, dendritic cell, macrophage and eosinophil function, and platelet aggregation. In this review, we will discuss the role of SLAM receptors and their adapters in T cell function, and we will examine the role of these receptors and their adapters in X-linked lymphoproliferative disease and their contribution to disease susceptibility in systemic lupus erythematosus.
Figures
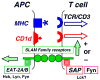
Fig. 1
SLAM family receptors in the immune synapse. The mostly homophilic interactions between SLAMF receptors subserve their role as costimulatory molecules. The binding of the SLAMF receptors to their ligands induces tyrosine phosphorylation of ITSM motifs in their cytoplasmic tail to which the adapters SAP or EAT-2 bind subsequently. SAP is mostly expressed by T cells, whereas EAT-2 is primarily expressed in APC
Fig. 2
Cytoplasmic tails of human SLAM receptors and the functional ITSM motifs and other docking sites. Cytoplasmic tails of human full-length SLAMF1 and SLAMF3-7 contain several ITSM motifs (tyrosine (Y) in red) which are docking sites for the SAP and EAT-2 adapters. Additional tyrosines are in the cytoplasmic tail of the SLAMF molecules (Y in black) are involved in the association with other effector molecules, e.g., SLAMF3 binds to the adaptor molecules AP-2 and Grb-2. Numbers indicate the tyrosine position relative to the molecule's N-terminal amino acid accession numbers between brackets correspond to Ensembl ID
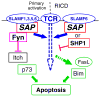
Fig. 3
A model depicting how apoptosis in CD8+ T cells is controlled by SAP in a Fyn-dependent and Fyn-independent manner. Signaling of SLAMF receptors through SAP binding and subsequent recruitment of Fyn fine-tunes the strength of the TCR signals and contributes in the p73-mediated way of apoptosis during primary T cell responses. In this model, activated Fyn could block the ubiquitin ligase Itch and therefore inhibit the degradation of p73. During the re-activation phase of T cells, SLAMF receptors, e.g., SLAMF6, might promote apoptosis by a SAP-dependent but Fyn-independent mechanism. In this model, SAP dislodges the phosphatase SHP1 from the cytoplasmic tail of SLAM molecules, which facilitates a stronger level of TCR signals sufficient to induce the transcription of RICD mediators such as FasL and Bim. Rectangles: intracellular signaling molecules, ellipses: membrane receptors or their secreted forms (FasL)
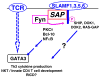
Fig. 4
Signal transduction by SLAM family surface receptors in CD4+ T cells. During formation of the immune synapse, clustering of SLAMF receptors brings SAP to its cytoplasmic tails and mediates recruitment and activation of Fyn. Thus, SLAMF receptors modulate TCR signaling by inducing a sustained recruitment of PKCθ and Bcl-10, which in turn leads to the activation of NF-κB and consequently to the production of Th2 cytokines and participation in NKT/innate CD4 T cell development. Phosphorylated tyrosines in the SLAMF cytoplasmic tails triggers the association with SHIP, docking protein 1 (DOK1), DOK2, and RAS-GAP, which may participate in these processes
Similar articles
- Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes.
Veillette A, Dong Z, Latour S. Veillette A, et al. Immunity. 2007 Nov;27(5):698-710. doi: 10.1016/j.immuni.2007.11.005. Immunity. 2007. PMID: 18031694 Review. - Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules.
Ma CS, Nichols KE, Tangye SG. Ma CS, et al. Annu Rev Immunol. 2007;25:337-79. doi: 10.1146/annurev.immunol.25.022106.141651. Annu Rev Immunol. 2007. PMID: 17201683 Review. - Role of SLAM-associated protein in the pathogenesis of autoimmune diseases and immunological disorders.
Furukawa H, Tohma S, Kitazawa H, Komori H, Nose M, Ono M. Furukawa H, et al. Arch Immunol Ther Exp (Warsz). 2010 Feb;58(1):37-44. doi: 10.1007/s00005-009-0060-7. Epub 2010 Jan 5. Arch Immunol Ther Exp (Warsz). 2010. PMID: 20049647 Review. - Functional requirements for interactions between CD84 and Src homology 2 domain-containing proteins and their contribution to human T cell activation.
Tangye SG, Nichols KE, Hare NJ, van de Weerdt BC. Tangye SG, et al. J Immunol. 2003 Sep 1;171(5):2485-95. doi: 10.4049/jimmunol.171.5.2485. J Immunol. 2003. PMID: 12928397 - Importance and mechanism of 'switch' function of SAP family adapters.
Veillette A, Dong Z, Pérez-Quintero LA, Zhong MC, Cruz-Munoz ME. Veillette A, et al. Immunol Rev. 2009 Nov;232(1):229-39. doi: 10.1111/j.1600-065X.2009.00824.x. Immunol Rev. 2009. PMID: 19909367
Cited by
- SAP modulates B cell functions in a genetic background-dependent manner.
Detre C, Yigit B, Keszei M, Castro W, Magelky EM, Terhorst C. Detre C, et al. Immunol Lett. 2013 Jun;153(1-2):15-21. doi: 10.1016/j.imlet.2013.06.003. Epub 2013 Jun 24. Immunol Lett. 2013. PMID: 23806511 Free PMC article. - Therapeutic Targets of Monoclonal Antibodies Used in the Treatment of Cancer: Current and Emerging.
Effer B, Perez I, Ulloa D, Mayer C, Muñoz F, Bustos D, Rojas C, Manterola C, Vergara-Gómez L, Dappolonnio C, Weber H, Leal P. Effer B, et al. Biomedicines. 2023 Jul 24;11(7):2086. doi: 10.3390/biomedicines11072086. Biomedicines. 2023. PMID: 37509725 Free PMC article. Review. - Gene-function studies in systemic lupus erythematosus.
Crispín JC, Hedrich CM, Tsokos GC. Crispín JC, et al. Nat Rev Rheumatol. 2013 Aug;9(8):476-84. doi: 10.1038/nrrheum.2013.78. Epub 2013 Jun 4. Nat Rev Rheumatol. 2013. PMID: 23732569 Review. - SLAMF6 deficiency augments tumor killing and skews toward an effector phenotype revealing it as a novel T cell checkpoint.
Hajaj E, Eisenberg G, Klein S, Frankenburg S, Merims S, Ben David I, Eisenhaure T, Henrickson SE, Villani AC, Hacohen N, Abudi N, Abramovich R, Cohen JE, Peretz T, Veillette A, Lotem M. Hajaj E, et al. Elife. 2020 Mar 3;9:e52539. doi: 10.7554/eLife.52539. Elife. 2020. PMID: 32122464 Free PMC article. - Expression patterns of signaling lymphocytic activation molecule family members in peripheral blood mononuclear cell subsets in patients with systemic lupus erythematosus.
Karampetsou MP, Comte D, Kis-Toth K, Kyttaris VC, Tsokos GC. Karampetsou MP, et al. PLoS One. 2017 Oct 11;12(10):e0186073. doi: 10.1371/journal.pone.0186073. eCollection 2017. PLoS One. 2017. PMID: 29020082 Free PMC article.
References
- Wang N, Morra M, Wu C, Gullo C, Howie D, Coyle T, Engel P, Terhorst C. CD150 is a member of a family of genes that encode glycoproteins on the surface of hematopoietic cells. Immunogenetics. 2001;53:382–394. - PubMed
- Tovar V, del Valle J, Zapater N, Martin M, Romero X, Pizcueta P, Bosch J, Terhorst C, Engel P. Mouse novel Ly9: a new member of the expanding CD150 (SLAM) family of leukocyte cell-surface receptors. Immunogenetics. 2002;54:394–402. - PubMed
- Fraser CC, Howie D, Morra M, Qiu Y, Murphy C, Shen Q, Gutierrez-Ramos JC, Coyle A, Kingsbury GA, Terhorst C. Identification and characterization of SF2000 and SF2001, two new members of the immune receptor SLAM/CD2 family. Immunogenetics. 2002;53:843–850. - PubMed
- Yokoyama S, Staunton D, Fisher R, Amiot M, Fortin JJ, Thorley-Lawson DA. Expression of the Blast-1 activation/adhesion molecule and its identification as CD48. J Immunol. 1991;146:2192–2200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK073339-03/DK/NIDDK NIH HHS/United States
- R01 DK073339/DK/NIDDK NIH HHS/United States
- AI-065687/AI/NIAID NIH HHS/United States
- AI-076210/AI/NIAID NIH HHS/United States
- P01 AI065687/AI/NIAID NIH HHS/United States
- R56 AI015066/AI/NIAID NIH HHS/United States
- P01 AI076210/AI/NIAID NIH HHS/United States
- R01 AI015066/AI/NIAID NIH HHS/United States
- AI-15066/AI/NIAID NIH HHS/United States
- P01 AI035714/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous