Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice - PubMed (original) (raw)
. 2010 Feb 10;30(6):2017-24.
doi: 10.1523/JNEUROSCI.5693-09.2010.
Dmitry V Vasilyev, Maria Beatriz Goncalves, Fiona V Howell, Carl Hobbs, Melina Reisenberg, Ru Shen, Mei-Yi Zhang, Brian W Strassle, Peimin Lu, Lilly Mark, Michael J Piesla, Kangwen Deng, Evguenia V Kouranova, Robert H Ring, Garth T Whiteside, Brian Bates, Frank S Walsh, Gareth Williams, Menelas N Pangalos, Tarek A Samad, Patrick Doherty
Affiliations
- PMID: 20147530
- PMCID: PMC6634037
- DOI: 10.1523/JNEUROSCI.5693-09.2010
Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice
Ying Gao et al. J Neurosci. 2010.
Abstract
Endocannabinoids (eCBs) function as retrograde signaling molecules at synapses throughout the brain, regulate axonal growth and guidance during development, and drive adult neurogenesis. There remains a lack of genetic evidence as to the identity of the enzyme(s) responsible for the synthesis of eCBs in the brain. Diacylglycerol lipase-alpha (DAGLalpha) and -beta (DAGLbeta) synthesize 2-arachidonoyl-glycerol (2-AG), the most abundant eCB in the brain. However, their respective contribution to this and to eCB signaling has not been tested. In the present study, we show approximately 80% reductions in 2-AG levels in the brain and spinal cord in DAGLalpha(-/-) mice and a 50% reduction in the brain in DAGLbeta(-/-) mice. In contrast, DAGLbeta plays a more important role than DAGLalpha in regulating 2-AG levels in the liver, with a 90% reduction seen in DAGLbeta(-/-) mice. Levels of arachidonic acid decrease in parallel with 2-AG, suggesting that DAGL activity controls the steady-state levels of both lipids. In the hippocampus, the postsynaptic release of an eCB results in the transient suppression of GABA-mediated transmission at inhibitory synapses; we now show that this form of synaptic plasticity is completely lost in DAGLalpha(-/-) animals and relatively unaffected in DAGLbeta(-/-) animals. Finally, we show that the control of adult neurogenesis in the hippocampus and subventricular zone is compromised in the DAGLalpha(-/-) and/or DAGLbeta(-/-) mice. These findings provide the first evidence that DAGLalpha is the major biosynthetic enzyme for 2-AG in the nervous system and reveal an essential role for this enzyme in regulating retrograde synaptic plasticity and adult neurogenesis.
Figures
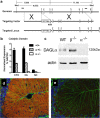
Figure 1.
Generation and validation of DAGLα−/− mice. a, Targeting strategy for DAGLα: genomic structure of the mouse DAGLα gene up to exon 8. Targeting vector replaces a portion of exon 1 with a selection cassette. Homologous integration is detected by Southern blotting using probes external to the targeting vector on both the 5′ and 3′ sides. Probes are indicated as solid lines above the genomic locus. Both probes detect an endogenous band of 16.4 kb in NdeI-cut genomic DNA. Homologous targeting creates bands of 8.6 and 11.1 kb when probed with 5′ or 3′ external probes, respectively. b, TaqMan analysis for DAGLα mRNA levels in cortex (CTX), cerebellum (Cb), and spinal cord (SC) from wt (+/+), DAGLα+/− (+/−), and DAGLα−/− (−/−) mice (n = 3–5/genotype). DAGLα expression is normalized as the percentage of GAPDH levels. Error bars indicate SEM. c, Western blot analysis of cerebellum tissues from wt (WT), DAGLα−/− (α−/−), and DAGLβ−/− (β−/−) mice demonstrates the absence of DAGLα protein in DAGLα−/− mice. Expression of actin in each sample is used as a loading control. In d, DAGLα expression is restricted to Purkinje cell dendrites in the cerebellum of adult wt mice (the red staining), with this staining lost in DAGLα−/− mice (e). Normal staining of GFAP, to highlight Bergman glial cells, was found in the wt and DAGLα−/− mice (the green staining in d and e). The sections were counterstained to show cell nuclei (in blue).
Figure 2.
Generation and validation of DAGLβ−/− mice. a, DAGLβ knock-out mice were generated from a Lexicon OmniBank ES cell clone OST195261, which contains a gene trap cassette insertion in the first exon of the mouse DAGLβ gene. LTR, Long terminal repeat; NEO, neomycin gene; pA, polyadenylation sequence; SV40tpA, SV40 triple polyadenylation sequence; PGK, phosphoglycerate kinase-1 promoter; BTK, Bruton tyrosine kinase. b, TaqMan analysis for DAGLβ mRNA levels in the cortex (CTX), cerebellum (Cb), spinal cord (SC), and liver (LI) from wt (+/+), DAGLβ+/− (+/−), and DAGLβ−/− (−/−) mice (n = 4/genotype). DAGLβ expression is normalized as the percentage of GAPDH levels. Error bars indicate SEM. c, Western blot analysis of cerebellum tissues from wt (WT), DAGLα−/− (α−/−), and DAGLβ−/− (β−/−) mice demonstrates the absence of DAGLβ protein in DAGLβ−/− mice.
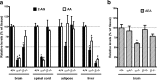
Figure 3.
2-AG, AA, and AEA levels in wt, DAGLα−/−, and DAGLβ−/− tissues. a, Levels of 2-AG and AA in brain, spinal cord, adipose, and liver from wt (+/+), DAGLα−/− (α−/−), and DAGLβ−/− (β−/−) mice (n = 4–13/genotype). Results are expressed as relative levels normalized to the same type of wt tissue. *,#p < 0.01, 2-AG, AA levels to the same type of wt tissue, t test. The levels of 2-AG (in nanomoles per gram) in wt tissues are 11.3 ± 1.0 in brain, 6.6 ± 1.0 in spinal cord, 0.5 ± 0.1 in adipose, and 4.6 ± 0.5 in liver. The levels of AA (in nanomoles per gram) in wt tissues are 93.3 ± 4.7 in brain, 57.7 ± 1.8 in spinal cord, 8.6 ± 0.7 in adipose, and 25.3 ± 2.2 in liver. b, Levels of AEA in the brain of wt (+/+) mice compared with mice with one (+/−) or zero (−/−) copies of the normal DAGLα/β locus (n = 3–8/genotype). Results are expressed as relative levels normalized to wt brain. *p < 0.01, to wt brain, t test. The levels of AEA (in picomoles per gram) in wt brain are 22.0 ± 2.1. Error bars indicate SEM.
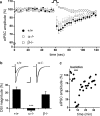
Figure 4.
DSI in the hippocampus of wt, DAGLα−/−, and DAGLβ−/− mice. a, DSI is absent in DAGLα−/− mice. Average time course for eIPSC amplitudes after depolarization in wt (+/+), DAGLα−/− (α−/−), and DAGLβ−/− (β−/−) mice. DSI for each genotype is averaged across all cells sampled, four to five DSI protocol repeats per sampled cell (+/+, n = 35; α−/−, n = 21; β−/−, n = 21). b, Peak DSI value is expressed as a percentage of depression in eIPSC amplitude after depolarization. Insets are representative recordings from a single CA1 neuron in wt (+/+), DAGLα−/− (α−/−), and DAGLβ−/− (β−/−) mice: the average of eIPSC traces (n = 4–5) just before DSI versus the average of eIPSC traces at the peak of DSI (second trace after 5 s depolarization; n = 4–5). Scale bar, 50 ms, 400 pA. *** p < .001, one-way ANOVA. Error bars indicate SEM. c, (RS)-Baclofen (10 μ
m
) displays a robust inhibition of eIPSC in DAGLα−/− mice, as indicating here the eIPSC recordings of a single CA1 neuron in a representative experiment; each point represents the average of six eIPSCs.
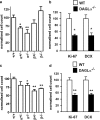
Figure 5.
DAGLα regulates neurogenesis in the hippocampus and SVZ. The relative levels of cell proliferation as indexed by the number of BrdU-positive cells present in the SVZ (a) or hippocampus (c) is shown for wt (+/+) mice compared with mice with one (+/−) or zero (−/−) copies of the normal DAGLα/β locus. Counts of Ki-67 and DCX in the SVZ (b) and DG in the hippocampus (d) in wt and DAGLα−/− mice. Results are normalized to control, and bars show SEM. **p < 0.01, *p < 0.05 (two-sided Student's t test). Mice are 7–10 weeks of age.
Similar articles
- Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex.
Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, Blat Y, Westphal RS, Zaczek R, Lewis DA, Gonzalez-Burgos G. Yoshino H, et al. J Physiol. 2011 Oct 15;589(Pt 20):4857-84. doi: 10.1113/jphysiol.2011.212225. Epub 2011 Aug 1. J Physiol. 2011. PMID: 21807615 Free PMC article. - DAGL-dependent endocannabinoid signalling: roles in axonal pathfinding, synaptic plasticity and adult neurogenesis.
Oudin MJ, Hobbs C, Doherty P. Oudin MJ, et al. Eur J Neurosci. 2011 Nov;34(10):1634-46. doi: 10.1111/j.1460-9568.2011.07831.x. Eur J Neurosci. 2011. PMID: 22103420 Review. - Diacylglycerol lipaseα (DAGLα) and DAGLβ cooperatively regulate the production of 2-arachidonoyl glycerol in autaptic hippocampal neurons.
Jain T, Wager-Miller J, Mackie K, Straiker A. Jain T, et al. Mol Pharmacol. 2013 Aug;84(2):296-302. doi: 10.1124/mol.113.085217. Epub 2013 Jun 7. Mol Pharmacol. 2013. PMID: 23748223 Free PMC article. - Brain regional cannabinoid CB(1) receptor signalling and alternative enzymatic pathways for 2-arachidonoylglycerol generation in brain sections of diacylglycerol lipase deficient mice.
Aaltonen N, Riera Ribas C, Lehtonen M, Savinainen JR, Laitinen JT. Aaltonen N, et al. Eur J Pharm Sci. 2014 Jan 23;51:87-95. doi: 10.1016/j.ejps.2013.08.035. Epub 2013 Sep 3. Eur J Pharm Sci. 2014. PMID: 24012970 - Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know.
Alger BE. Alger BE. J Physiol. 2012 May 15;590(10):2203-12. doi: 10.1113/jphysiol.2011.220855. Epub 2012 Jan 30. J Physiol. 2012. PMID: 22289914 Free PMC article. Review.
Cited by
- cAMP Signaling-Mediated Phosphorylation of Diacylglycerol Lipase α Regulates Interaction With Ankyrin-G and Dendritic Spine Morphology.
Yoon S, Myczek K, Penzes P. Yoon S, et al. Biol Psychiatry. 2021 Aug 15;90(4):263-274. doi: 10.1016/j.biopsych.2021.03.023. Epub 2021 Mar 26. Biol Psychiatry. 2021. PMID: 34099188 Free PMC article. - Cannabinoids and Tremor Induced by Motor-related Disorders: Friend or Foe?
Arjmand S, Vaziri Z, Behzadi M, Abbassian H, Stephens GJ, Shabani M. Arjmand S, et al. Neurotherapeutics. 2015 Oct;12(4):778-87. doi: 10.1007/s13311-015-0367-5. Neurotherapeutics. 2015. PMID: 26152606 Free PMC article. Review. - Therapeutic potential of monoacylglycerol lipase inhibitors.
Mulvihill MM, Nomura DK. Mulvihill MM, et al. Life Sci. 2013 Mar 19;92(8-9):492-7. doi: 10.1016/j.lfs.2012.10.025. Epub 2012 Nov 8. Life Sci. 2013. PMID: 23142242 Free PMC article. Review. - Self-tuning of inhibition by endocannabinoids shapes spike-time precision in CA1 pyramidal neurons.
Dubruc F, Dupret D, Caillard O. Dubruc F, et al. J Neurophysiol. 2013 Oct;110(8):1930-44. doi: 10.1152/jn.00099.2013. Epub 2013 Jul 31. J Neurophysiol. 2013. PMID: 23904493 Free PMC article. - Endocannabinoid biosynthetic enzymes regulate pain response via LKB1-AMPK signaling.
Chen M, Shin M, Ware TB, Donvito G, Muchhala KH, Mischel R, Mustafa MA, Serbulea V, Upchurch CM, Leitinger N, Akbarali HI, Lichtman AH, Hsu KL. Chen M, et al. Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2304900120. doi: 10.1073/pnas.2304900120. Epub 2023 Dec 18. Proc Natl Acad Sci U S A. 2023. PMID: 38109529 Free PMC article.
References
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzmán M, Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. - PubMed
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. - PubMed
- Bilsland JG, Haldon C, Goddard J, Oliver K, Murray F, Wheeldon A, Cumberbatch J, McAllister G, Munoz-Sanjuan I. A rapid method for the quantification of mouse hippocampal neurogenesis in vivo by flow cytometry. Validation with conventional and enhanced immunohistochemical methods. J Neurosci Methods. 2006;157:54–63. - PubMed
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous