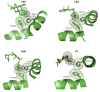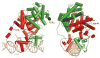Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics - PubMed (original) (raw)
Review
Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics
Pengxiang Huang et al. Annu Rev Physiol. 2010.
Abstract
As ligand-regulated transcription factors, the nuclear hormone receptors are nearly ideal drug targets, with internal pockets that bind to hydrophobic, drug-like molecules and well-characterized ligand-induced conformational changes that recruit transcriptional coregulators to promoter elements. Yet, due to the multitude of genes under the control of a single receptor, the major challenge has been the identification of ligands with gene-selective actions, impacting disease outcomes through a narrow subset of target genes and not across their entire gene-regulatory repertoire. Here, we summarize the concepts and work to date underlying the development of steroidal and nonsteroidal receptor ligands, including the use of crystal structures, high-throughput screens, and rational design approaches for finding useful therapeutic molecules. Difficulties in finding selective receptor modulators require a more complete understanding of receptor interdomain communications, posttranslational modifications, and receptor-protein interactions that could be exploited for target gene selectivity.
Figures
Figure 1
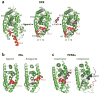
(a) The 12-helical canonical fold of nuclear receptor (NR) ligand-binding domains (LBDs) and the conformational changes in retinoid X receptor (RXR) associated with ligand binding and receptor activation. The unliganded RXR-LBD changes its protein conformation, including helix 12 (H12) (shown in red) positioning upon binding to an agonist (SR11237). The ligand-activated receptor uses H12, as well as additional features in H3 and H4, to promote the binding of short helical LXXLL (where L is leucine and X is any residue) elements from coactivator protein (shown in purple) (21). (b) H12 geometry and activation function-2 (AF-2) function is ligand dependent. NR agonists, such as the natural ligand 17β-estradiol (E2) in the estrogen receptor (ER), cause a conformation in the LBD, particularly in H12 (red), which supports the formation of a binding surface for the helical NR-box module of the coactivators. Antagonists such as raloxifene, through the extension group on the C-ring of their ligand, induce alternate conformations in the LBD in which H12 is displaced away from its agonist conformation where it normally supports NR-box binding. Instead, H12 moves into that site of coactivator motif, using its own helical LXXML sequence to mimic the interactions normally made by the LXXLL motifs (41, 42). (c) The ligand-mediated exchange of coactivators and corepressors on NRs. The peroxisome proliferator–activated receptor α (PPARα) binding to coactivator versus corepressor segments can be governed by specific ligands. Whereas agonist ligands such as GW731 recruit coactivator NR-box elements from coactivators (SRC-1, shown in purple) to PPARα LBD (PDB ID 2P54), some antagonist ligands, such as GW6471, prevent the AF-2 helix (H12, shown in red) from assuming the active conformation, resulting in a larger crevice that can accommodate a corepressor (SMRT, shown in blue) segment with the LXXXIXXXL motif (25).
Figure 2
Ligand-induced effects on helix 12 (H12) through a pi-cation activation switch. A number of nuclear receptors, such as those shown here, use a conserved switch involving an electrostatic interaction between the indole ring histidine on H10/H11 and a single aromatic side chain, typically a trytophan residue, on H12. An oxygen group from each receptor’s endogenous ligand [6-ethyl-CDCA for farnesoid X receptor (FXR); 24-25S-epoxycholesterol for liver X receptor β (LXRβ); 1α,25-dihydroxyvitamin D3 with vitamin D receptor (VDR); T3 with thyroid hormone receptor β (TRβ)] directly interacts with the histidine on H10/H11 but never directly contacts H12, nevertheless stabilizing H12 in the agonist conformation (26, 27, 109, 164).
Figure 3
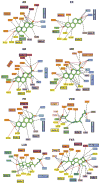
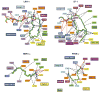
Close-up views of the ligand-binding interactions of nuclear receptors (NRs) that bind to steroidal molecules. Aside from farnesoid X receptor (FXR) binding to bile acids, the other receptors all position ring-A deep into the receptor pocket, where the 3-OH group forms hydrogen bonds with polar amino acid side chains. AR, androgen receptor; ER, estrogen receptor; GR, glucocorticoid receptor; LXR, liver X receptor; MR, mineralocorticoid receptor; PR, progesterone receptor; VDR, vitamin D receptor.
Figure 4
Close-up views of the ligand binding interactions of nuclear receptors (NRs) that bind to nonsteroidal molecules, including all-trans retinoic acid (ATRA), 9-cis retinoic acid (9 cRA), triiodothryronine (T3), and iron-porphyrin IX (heme). RAR, retinoic acid receptor; RXR, retinoid X receptor; TR, thyroid hormone receptor.
Figure 5
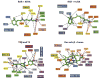
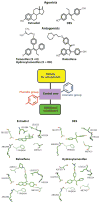
Principles of ligand design as agonists and antagonists for the estrogen receptor (ER). Estrogens (agonists) include the natural ligand E2 and the synthetic hormone diethylstilbestrol (DES). Tamoxifen/hydroxytamoxifen and raloxifene are antiestrogens and function more as SERMs (selective estrogen receptor modulators). Although mimicking some general features of the basic molecular structure in these ligands, including the central core, with an aromatic and phenolic group on either sides, additional moieties such as extensions along one of the rings can convert ligands from agonists to antagonists on the basis of the protrusion of this group disrupting the agonist conformation of H12. Close-up views of the pocket around these four ligands are provided at the bottom, showing that the conserved features of their chemical frame are recognized similarly within the ER pocket (–167).
Figure 6
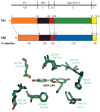
Estrogen receptor (ER)α and β have different tissue/cell distributions and differential affinities for ligands and nonoverlapping genes that they regulate. As a result, targeting these receptors differentially can lead to selective gene activation profiles. Shown are the relationships of their amino acid sequences, including the level of conservation (in parentheses). Within the pocket, there are only two residues that differ, yet molecules such as WAY-244 can show a very high preference for ERβ (53). A–F designate the segments of the receptor polypeptides.
Figure 7
Demonstration of how ligands can stretch and significantly reshape receptor ligand-binding domain (LBD) pockets. The three structures of the glucocorticoid receptor (GR) LBD pocket with dexamathasone (DEX), RU-486, and DAC indicate how the residues surrounding the ligands can undergo both side-chain and main-chain movements to nearly double the size of the pocket to fit with each of these steroidal molecules (–108).
Figure 8
Nuclear receptor interactions with lipid molecules. LRH-1 is shown with phosphotidyl glycerol, SF-1 with di-palmitoyl-3-SN-phosphotidylethanolamine, HNF4-α with myristic acid, and peroxisome proliferator–activated receptor γ (PPARγ) with 9-HODE (7, 8). LRH-1, liver receptor homolog-1; HNF-4, hepatocyte nuclear factor-4; SF-1, steroidogenic factor-1.
Figure 9
Overviews of the intact structures of the PPARγ-RXRα heterodimer on PPAR response element DNA. The PPARγ is in red, RXRα in blue, DNA in copper, coactivator LXXLL motifs in blue and orange, and the ligands in yellow. The PPARγ LBD makes interdomain contacts with all the other ordered domains in both polypeptides. An unexpected interface between the PPARγ LBD and both DBDs suggests that ligands at PPARγ may modulate DNA site affinities (157). DBD, DNA-binding domain; LBD, ligand-binding domain; PPARγ, peroxisome proliferator–activated receptor γ; RXR, retinoid X receptor.
Figure 10
Superposition of the binding modes of three peroxisome proliferator–activated receptor γ (PPARγ) synthetic ligands. Rosiglitazone is a full agonist, BVT.13 is a partial agonist, and GW9662 is a suicide inhibitor that binds to the receptor pocket. Together, the superposition shows the Y-shaped inducible fit possible in this receptor pocket (157).
Similar articles
- Structure and function of the human nuclear xenobiotic receptor PXR.
Carnahan VE, Redinbo MR. Carnahan VE, et al. Curr Drug Metab. 2005 Aug;6(4):357-67. doi: 10.2174/1389200054633844. Curr Drug Metab. 2005. PMID: 16101574 Review. - Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action.
Shibata H, Spencer TE, Oñate SA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW. Shibata H, et al. Recent Prog Horm Res. 1997;52:141-64; discussion 164-5. Recent Prog Horm Res. 1997. PMID: 9238851 Review. - A nuclear receptor corepressor modulates transcriptional activity of antagonist-occupied steroid hormone receptor.
Zhang X, Jeyakumar M, Petukhov S, Bagchi MK. Zhang X, et al. Mol Endocrinol. 1998 Apr;12(4):513-24. doi: 10.1210/mend.12.4.0089. Mol Endocrinol. 1998. PMID: 9544987 - [Hormonal regulation of gene transcription--nuclear hormone receptors as ligand-activated transcription factors].
Hatina J, Reischig J. Hatina J, et al. Cesk Fysiol. 2000 May;49(2):61-72. Cesk Fysiol. 2000. PMID: 10953507 Review. Czech. - Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes.
Wang H, LeCluyse EL. Wang H, et al. Clin Pharmacokinet. 2003;42(15):1331-57. doi: 10.2165/00003088-200342150-00003. Clin Pharmacokinet. 2003. PMID: 14674787 Review.
Cited by
- Investigation of a Radio-Iodinated Alpha-Mangostin for Targeting Estrogen Receptor Alpha (ERα) in Breast Cancer: In Silico Design, Synthesis, and Biological Evaluation.
Muchtaridi M, Nurhidayah W, Fakih TM, Kannaka K, Suzuki H, Subroto T, Uehara T. Muchtaridi M, et al. Drug Des Devel Ther. 2024 Oct 9;18:4511-4526. doi: 10.2147/DDDT.S479447. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39399125 Free PMC article. - Combining graph neural networks and transformers for few-shot nuclear receptor binding activity prediction.
Torres LHM, Arrais JP, Ribeiro B. Torres LHM, et al. J Cheminform. 2024 Sep 27;16(1):109. doi: 10.1186/s13321-024-00902-4. J Cheminform. 2024. PMID: 39334272 Free PMC article. - Nuclear Receptors and the Hidden Language of the Metabolome.
Chen Y, Anderson MT, Payne N, Santori FR, Ivanova NB. Chen Y, et al. Cells. 2024 Jul 31;13(15):1284. doi: 10.3390/cells13151284. Cells. 2024. PMID: 39120315 Free PMC article. Review. - Mechanism of antagonist ligand binding to REV-ERBα.
Rahman MH, Hegazy L. Rahman MH, et al. Sci Rep. 2024 Apr 10;14(1):8401. doi: 10.1038/s41598-024-58945-4. Sci Rep. 2024. PMID: 38600172 Free PMC article. - Restricted effects of androgens on glucocorticoid signaling in the mouse prefrontal cortex and midbrain.
Amaya JM, Sips HCM, Viho EMG, Kroon J, Meijer OC. Amaya JM, et al. Front Endocrinol (Lausanne). 2024 Jan 18;14:1292024. doi: 10.3389/fendo.2023.1292024. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38303978 Free PMC article.
References
- McEwan IJ. Nuclear receptors: one big family. Methods Mol Biol. 2009;505:3–18. - PubMed
- Nagy L, Schwabe JW. Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci. 2004;29:317–24. - PubMed
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials