TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients - PubMed (original) (raw)
TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients
Courtney A Crane et al. Neuro Oncol. 2010 Jan.
Abstract
The activating receptor NKG2D, expressed by natural killer (NK) cells and CD8(+) T cells, has a role in the specific killing of transformed cells. We examined NKG2D expression in patients with glioblastoma multiforme and found that NKG2D was downregulated on NK cells and CD8(+) T cells. Expression of NKG2D on lymphocytes significantly increased following tumor resection and correlated with an increased ability to kill NKG2D ligand-positive tumor targets. Despite the presence of soluble NKG2D ligands in the sera of glioblastoma patients, NKG2D downregulation was primarily caused by tumor-derived tumor growth factor-beta, suggesting that blocking of this cytokine may have therapeutic benefit.
Figures
Fig. 1.
Expression of the activating receptor NKG2D is decreased on NK and CD8+ T cells in patients with glioblastoma multiforme (GBM). (A) Peripheral blood lymphocytes (PBLs) isolated from patients with either GBM or MNG were isolated prior to tumor resection. CD8+ T lymphocytes were defined as CD3+, CD8+ cells: NK cells were defined as CD3−, CD56+ (left panels). After gating, cells were analyzed for the expression of NKG2D relative to the expression levels in patients with MNG. (B) PBL and tumor-infiltrating lymphocytes (TILs) were isolated from GBM patients (n = 23) and analyzed for the expression of NKG2D on CD3+, CD8+ cells, gated as shown in the top panels, and compared with those isolated from patients with MNG (n = 12). (C) PBL from GBM patients were analyzed for expression levels of NKG2D on NK and CD8+ T cells before and after a significant surgical reduction in tumor burden as confirmed with MRI. Each point represents one patient.
Fig. 2.
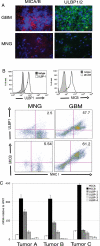
GBM tumor cells express NKG2D ligands on the surface. (A) Immunofluorescence staining of 10-µm frozen tissue sections with monoclonal antibody cocktails with either anti-MICA (clone M673) and anti-MICB (clone M362) (left panels, red) or anti-ULBP1 (clone M295) and anti-ULBP-2 (M310) (right panels, green) in GBM or MNG tumor sections. Nuclei were stained with DAPI (blue). Images are representative of 11 GBM samples analyzed. (B) Histograms: single-cell suspensions of tumor tissue from either GBM patients were stained PE-conjugated anti-MICA/B (clone 6D4) or FITC-conjugated anti-ULBP-1 (clone 170818) or the relevant isotype controls. Histograms represent gating of CD45−, MHC class I+ cells (tumor cells). Dot plots: GBM or MNG patient single-cell suspensions were stained with anti-MICA/B or anti-ULBP-1 and gated on CD45− cells. Data are representative of 18 GBM samples and 11 MNG samples analyzed. (C), mRNA expression levels of NKG2D ligands in CD45−, MHC class I+ tumor cells. mRNA was analyzed for the presence of NKG2D ligands and expressed as mRNA units relative to the expression of the housekeeping gene HPRT.
Fig. 3.
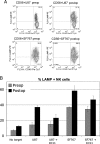
NK cells lyse tumor cell targets in an NKG2D-dependent manner more efficiently after tumor resection. (A) NK cells were isolated from PBLs and activated overnight with IL-2. NKG2D ligand-positive tumor cell lines U87 (top panels) and SF767 (bottom panels) were cocultured for 3 h with NK cells isolated from patients before and after tumor resection (preoperatively and post-operatively). Target cell recognition was measured by expression of LAMP-1 (CD107), a marker for NK cell degranulation, and lymphocytes (CD45+) were distinguished from tumor cells based on CD45 expression. (B) CD107 staining of NK cells following coculture. Anti-NKG2D (clone 1D11) was added to inhibit the interaction between NKG2D and its ligands. Data shown are from a representative experiment (of three) performed in triplicate.
Fig. 4.
GBM patient sera contain soluble NKG2D ligands but decreased expression of NKG2D is at the level of transcription and mediated by TGF-β. (A) Patient sera from 41 GBM patients and 30 MNG patients were analyzed by ELISA for soluble MICB. Each point represents one patient. The bottom panel represents the percentage of GBM or MNG patients with soluble MICB levels within a given range. (B) NK cells and CD8+ T cells were isolated using CD56 or CD8 cell selection and whole-cell mRNA reverse transcribed before and after tumor resection. Amount of NKG2D mRNA was determined relative to HPRT and expressed as a ratio of detected levels after surgery: levels before surgery. A ratio of >1 represents an increase in NKG2D levels following tumor resection. (C) NK cells from an MNG patient were cocultured with patient sera containing 4.8 ng/mL soluble MICB for 48 hours in the presence of 10 µg/mL of TGF-β–receptor blocking antibody. As a positive control, 1 ng/mL recombinant TGF-β was added to PBLs and blocked using TGF-β receptor antibody. Surface expression of NKG2D was then analyzed by flow cytometry.
Similar articles
- B7H6 is the predominant activating ligand driving natural killer cell-mediated killing in patients with liquid tumours: evidence from clinical, in silico, in vitro, and in vivo studies.
Lee S, Chae SJ, Jang IH, Oh SC, Kim SM, Lee SY, Kim JH, Ko J, Kim HJ, Song IC, Kim JK, Kim TD. Lee S, et al. EBioMedicine. 2024 Dec;110:105459. doi: 10.1016/j.ebiom.2024.105459. Epub 2024 Nov 22. EBioMedicine. 2024. PMID: 39579618 Free PMC article. - 4-1BB regulates NKG2D costimulation in human cord blood CD8+ T cells.
Kim YJ, Han MK, Broxmeyer HE. Kim YJ, et al. Blood. 2008 Feb 1;111(3):1378-86. doi: 10.1182/blood-2007-01-069450. Epub 2007 Nov 16. Blood. 2008. PMID: 18024793 Free PMC article. - NKG2D receptor signaling enhances cytolytic activity by virus-specific CD8+ T cells: evidence for a protective role in virus-induced encephalitis.
Walsh KB, Lanier LL, Lane TE. Walsh KB, et al. J Virol. 2008 Mar;82(6):3031-44. doi: 10.1128/JVI.02033-07. Epub 2007 Dec 26. J Virol. 2008. PMID: 18160433 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Killer dendritic cells and their potential for cancer immunotherapy.
Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Larmonier N, et al. Cancer Immunol Immunother. 2010 Jan;59(1):1-11. doi: 10.1007/s00262-009-0736-1. Epub 2009 Jul 18. Cancer Immunol Immunother. 2010. PMID: 19618185 Free PMC article. Review.
Cited by
- Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry.
Rühle PF, Fietkau R, Gaipl US, Frey B. Rühle PF, et al. Int J Mol Sci. 2016 Aug 11;17(8):1316. doi: 10.3390/ijms17081316. Int J Mol Sci. 2016. PMID: 27529227 Free PMC article. - Immunotherapy for glioblastoma: current state, challenges, and future perspectives.
Liu Y, Zhou F, Ali H, Lathia JD, Chen P. Liu Y, et al. Cell Mol Immunol. 2024 Dec;21(12):1354-1375. doi: 10.1038/s41423-024-01226-x. Epub 2024 Oct 15. Cell Mol Immunol. 2024. PMID: 39406966 Free PMC article. Review. - The Immune Microenvironment in Pancreatic Cancer.
Huber M, Brehm CU, Gress TM, Buchholz M, Alashkar Alhamwe B, von Strandmann EP, Slater EP, Bartsch JW, Bauer C, Lauth M. Huber M, et al. Int J Mol Sci. 2020 Oct 3;21(19):7307. doi: 10.3390/ijms21197307. Int J Mol Sci. 2020. PMID: 33022971 Free PMC article. Review. - Immunoediting Dynamics in Glioblastoma: Implications for Immunotherapy Approaches.
Amin T, Hossain A, Jerin N, Mahmud I, Rahman MA, Rafiqul Islam SM, Islam SMBU. Amin T, et al. Cancer Control. 2024 Jan-Dec;31:10732748241290067. doi: 10.1177/10732748241290067. Cancer Control. 2024. PMID: 39353594 Free PMC article. Review. - ROS regulation in gliomas: implications for treatment strategies.
Yang YC, Zhu Y, Sun SJ, Zhao CJ, Bai Y, Wang J, Ma LT. Yang YC, et al. Front Immunol. 2023 Dec 7;14:1259797. doi: 10.3389/fimmu.2023.1259797. eCollection 2023. Front Immunol. 2023. PMID: 38130720 Free PMC article. Review.
References
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. - PubMed
- Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science. 1974;183:534–536. - PubMed
- Dighe AS, Richards E, Old LJ, et al. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. - PubMed
- Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. - PubMed
- Groh V, Rhinehart R, Randolph-Habecker J, et al. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials