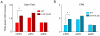Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety - PubMed (original) (raw)
Comparative Study
Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety
Avishek Adhikari et al. Neuron. 2010.
Abstract
The ventral hippocampus, unlike its dorsal counterpart, is required for anxiety-like behavior. The means by which it acts are unknown. We hypothesized that the hippocampus synchronizes with downstream targets that influence anxiety, such as the medial prefrontal cortex (mPFC). To test this hypothesis, we recorded mPFC and hippocampal activity in mice exposed to two anxiogenic arenas. Theta-frequency activity in the mPFC and ventral, but not dorsal, hippocampus was highly correlated at baseline, and this correlation increased in both anxiogenic environments. Increases in mPFC theta power predicted avoidance of the aversive compartments of each arena and were larger in serotonin 1A receptor knockout mice, a genetic model of increased anxiety-like behavior. These results suggest a role for theta-frequency synchronization between the ventral hippocampus and the mPFC in anxiety. They are consistent with the notion that such synchronization is a general mechanism by which the hippocampus communicates with downstream structures of behavioral relevance.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1. Characterization of LFPs from the mPFC, vHPC and dHPC in the familiar arena
(A) Traces of simultaneously recorded local field potentials from the mPFC, vHPC and dHPC in a mouse exploring the familiar arena. Raw traces are plotted in grey and theta filtered traces are overlaid in black. Underlines indicate a period robust theta activity in mPFC with minimal theta in the vHPC (*) and a period of robust theta-range activity in both mPFC and vHPC (**). Calibration: horizontal bar:1 s, vertical bar: 0.5 mV for mPFC and vHPC and 2.5 mV for dHPC trace. (B) Power spectra for different speed ranges for mPFC, vHPC and dHPC. Note that the peak centered at the theta range increases with higher speeds in all three areas. Also note the different scale on dHPC figure; theta power is much higher in dHPC than in vHPC and mPFC. Spectra are averages of 13 animals. (C) Coherence averaged across animals for mPFC-vHPC (blue), mPFC-dHPC (purple) and vHPC-dHPC (grey) recorded in the 7-15 cm/s speed range. Note that mPFC-vHPC coherence is higher than mPFC-dHPC for all frequencies. Shaded areas indicate 95% confidence intervals and the red line at the bottom shows the coherence expected by chance (p<0.05). See also Figures S1, S7 and S8.
Figure 2. Power correlations and phase coherence across areas
(A) Representative examples of theta power correlation scatter plots for vHPC-mPFC, dHPC-mPFC and vHPC-dHPC from a 10 minute recording session in the familiar arena. Each data point represents the sum of theta power during a 2.6 s window. (B) Averages of the linear correlation coefficients of theta (left corner) and gamma (right corner) power across 13 animals for vHPC-mPFC, dHPC-mPFC and vHPC-dHPC. Error bars are ± s.e.m. *p<0.01 for paired t-tests on the Fisher’s Z-transformed r values compared to mPFC-vHPC. (C) Representative histogram of theta phase differences. Instantaneous theta phase of two signals were subtracted from each other and the difference in theta phase was plotted as a histogram, for mPFC-vHPC (black), mPFC-dHPC (dark grey) and dHPC-vHPC (light grey). Narrower peaks in the histogram indicate a more consistent phase relationship. (D) Width of theta phase difference histogram at half of the peak height averaged across 13 animals for vHPC-mPFC (right panel), dHPC-mPFC (center panel) and vHPC-dHPC (left panel). Error bars are ± s.e.m. *p<0.01 for t-test comparing mPFC-vHPC to mPFC-dHPC.
Figure 3. Theta power correlation between mPFC and vHPC increases in the EPM and open field
(A) Representative example of theta power correlation plot in the familiar arena between mPFC and vHPC (upper panel) and dHPC (lower panel). (B) Theta power correlation plot in the open field from the same animal as in Fig. 4a between mPFC and vHPC (upper panel) and the dHPC (lower panel). Note the increase in mPFC-vHPC linear correlation r2 compared to Figure 4 A. Changes in averaged r2 of theta power correlations between in the familiar arena and open field (C) and EPM D) for mPFC-vHPC (left panel), mPFC-dHPC (middle panel) and dHPC-vHPC (right panel). Bars are averages of data from 13 animals, error bars are ± s.e.m. *p<0.05 for a paired t-test for the Fisher’s Z transformed r values. See also Figures S2, S3 and S4.
Figure 4. Multiunit phase-locking to mPFC and vHPC theta increases in the open field
(A) Representative examples of the distribution of preferred phases of multiunit activity recorded in the mPFC relative to local (upper panels) and vHPC (lower panels) theta oscillations in the familiar arena (black histograms) and the open field (red histograms). (B) Mean +/− S.E.M. of MRL values in the open field relative to the familiar environment for multiunit recordings to mPFC (left bar), vHPC (middle) and dHPC (right) theta oscillations. Note that the MRL in the open field is larger than in the familiar arena, indicating more robust phase-locking to both mPFC and vHPC theta oscillations. (C-E). mPFC units phase lock best to local theta of the present (C) and hippocampal theta o f the past (D,E). Color-coded plots show changes in MRL values for multiunit recordings after spikes are shifted in time relative to theta oscillations of mPFC (C), vHPC (D) and dHPC (E). Higher MRL values correspond to warmer colors. Each row corresponds to one multiunit recording. Rows are arranged according to the temporal offsets that produce maximal phase locking. Upper rows correspond to multiunit recordings that phase lock most robustly with large negative shifts, i.e., maximal phase locking to theta of the past. Histograms showing the population distribution of the temporal offsets with highest phase locking are shown on the right. The population mean is indicated by red arrows. Note that on average spikes in the mPFC are most strongly phase locked to hippocampal theta of theta of the past. Only recordings that were significantly phase locked (by Rayleigh’s test for circular uniformity, Bonferroni corrected p< 0.05/40) in at least one temporal shift were used. n=28-30 multiunit recordings. *p<0.05 for a paired Wilcoxon’s signed rank test on MRL values. (F) Example of crosscorrelation of mPFC and vHPC theta power. Note that the crosscorrelation peaks at a negative lag, indicating that theta power changes occur first in the vHPC and then in the mPFC. Instantaneous power was calculated through the Hilbert transform. (G) Histogram showing the distribution of lags with maximal crosscorrelation across animals. The median lag is significantly different from zero (−8 ms, p<0.05, signrank test). Only segments of data where vHPC theta power was greater than the mean vHPC theta power for a given session were used. The population mean is indicated by a red arrow.
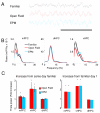
Figure 5. Theta power in the mPFC and dHPC increases during exposure to the EPM and open field
(A) Representative traces of mPFC LFPs recorded from the same animal in the familiar arena, open field and EPM. Calibration: 1s. (B) Examples of representative power spectra in the familiar arena (black traces), open field (red) and EPM (blue) from LFPs of the mPFC (left panel), vHPC (center) and dHPC (right). Mean power was calculated using the Welch method with s.e.m. (dashed lines) calculated across windows. (C) Left panel: Fold increases in theta power relative to the familiar arena exposures obtained in the same day as the open field (red bars) and in the EPM (blue bars) recordings. Right panel: Same as left panel, but relative to the first day of exposure to the familiar environment. All data are taken from epochs in which animals were running consistently in the 7-15 cm/s speed range. See also Figures S5 and S9.
Figure 6. mPFC theta power is increased specifically in the safe zones of the anxiogenic arenas
(A) Theta power increases in the mPFC, vHPC and dHPC during navigation of the periphery (dark red) and center (bright red) of the open field. (B) Theta power increase in each area during navigation of the closed arms (dark blue), open arms (medium blue), and center (light blue) of the elevated plus maze. n=18 and 12 for the open field and EPM, respectively. All data are from epochs in which animals were running consistently in the 7-15 cm/s speed range. Fold increases are relative to theta power in the familiar arena exposure on the same day. Error bars are ± s.e.m. *p<0.05, for a paired Wilcoxon’s signed rank test.
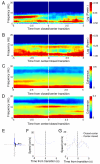
Figure 7. mPFC theta power and mPFC-vHPC coherence increase prior to leaving the closed arms
(A) Average mPFC spectrogram of all closed arm to center transitions in the EPM, centered at the transition point (time=0 sec). (B) Same as (A), but for center to closed arm transitions. Note sharp changes in mPFC theta power occur 2-3 s before the animal enters a new compartment of the maze. (C) Average mPFC-vHPC coherence centered at the closed to center transition. (D) Same as (C), but for center to closed transitions. (E) Example track of a closed to center transition. 10 seconds of movement (blue trace) centered at the transition is shown. Grey trace tracks the position of the mouse in the entire session. The black bar indicates the position of the transition, and the arrow shows the direction of movement. (F) Speed across time for the example transition shown in (E). (G) Average speed for both closed to center and center to closed transitions is shown.
Figure 8. mPFC theta power increases in the EPM and the open field correlate with behavioral measures of anxiety
(A,B) Scatter plots of mPFC fold theta increase relative to the familiar arena against % time spent in the center of the open field, (A) and % time in the open arms for the EPM, (B). (C,D) Plots of fold increases in theta power in the periphery of the open field and in the closed arms of the EPM relative to the familiar environment recording of the same day, as a function of anxiety-associated behaviors in the open field (C) and the EPM (D). Lower panels show movement tracks as heat maps for selected points, indicated by arrows. In the maps of the EPM, open arms are vertically oriented. Fold theta power changes were calculated from epochs in which animals were running consistently in the 7-15 cm/s speed range.
Figure 9. 5-HT1A knockouts (5-HT1A KO) have a higher increase in mPFC theta power in the EPM and the open field relative to wildtype (WT) mice
Bar graphs of average fold theta increase in the mPFC (left), vHPC (center) and dHPC (right), for the open field (red bars, upper panel) and EPM (blue bars, lower panel). Bars represent averages of 7 WT (clear bars) and 7 5-HT1A KO (thatched bars) mice. Error bars are ± s.e.m. *p<0.05 for a paired Wilcoxon’s signed rank test comparing the fold theta increases of WT and 5-HT1A KO animals. Fold theta power changes were calculated from epochs in which animals were running consistently in the 7-15 cm/s speed range.
Similar articles
- Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity.
Adhikari A, Topiwala MA, Gordon JA. Adhikari A, et al. Neuron. 2011 Sep 8;71(5):898-910. doi: 10.1016/j.neuron.2011.07.027. Neuron. 2011. PMID: 21903082 Free PMC article. - Hippocampal-Prefrontal Theta Transmission Regulates Avoidance Behavior.
Padilla-Coreano N, Canetta S, Mikofsky RM, Alway E, Passecker J, Myroshnychenko MV, Garcia-Garcia AL, Warren R, Teboul E, Blackman DR, Morton MP, Hupalo S, Tye KM, Kellendonk C, Kupferschmidt DA, Gordon JA. Padilla-Coreano N, et al. Neuron. 2019 Nov 6;104(3):601-610.e4. doi: 10.1016/j.neuron.2019.08.006. Epub 2019 Sep 11. Neuron. 2019. PMID: 31521441 Free PMC article. - Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion.
O'Neill PK, Gordon JA, Sigurdsson T. O'Neill PK, et al. J Neurosci. 2013 Aug 28;33(35):14211-24. doi: 10.1523/JNEUROSCI.2378-13.2013. J Neurosci. 2013. PMID: 23986255 Free PMC article. - Theta Oscillations Through Hippocampal/Prefrontal Pathway: Importance in Cognitive Performances.
Soltani Zangbar H, Ghadiri T, Seyedi Vafaee M, Ebrahimi Kalan A, Fallahi S, Ghorbani M, Shahabi P. Soltani Zangbar H, et al. Brain Connect. 2020 May;10(4):157-169. doi: 10.1089/brain.2019.0733. Epub 2020 May 7. Brain Connect. 2020. PMID: 32264690 Review. - The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: a model for schizophrenia.
Jodo E. Jodo E. J Physiol Paris. 2013 Dec;107(6):434-40. doi: 10.1016/j.jphysparis.2013.06.002. Epub 2013 Jun 17. J Physiol Paris. 2013. PMID: 23792022 Review.
Cited by
- Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders.
Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Kheirbek MA, et al. Nat Neurosci. 2012 Dec;15(12):1613-20. doi: 10.1038/nn.3262. Epub 2012 Nov 27. Nat Neurosci. 2012. PMID: 23187693 Free PMC article. Review. - The hippocampus and exploration: dynamically evolving behavior and neural representations.
Johnson A, Varberg Z, Benhardus J, Maahs A, Schrater P. Johnson A, et al. Front Hum Neurosci. 2012 Jul 25;6:216. doi: 10.3389/fnhum.2012.00216. eCollection 2012. Front Hum Neurosci. 2012. PMID: 22848196 Free PMC article. - Stress-induced vagal activity influences anxiety-relevant prefrontal and amygdala neuronal oscillations in male mice.
Okonogi T, Kuga N, Yamakawa M, Kayama T, Ikegaya Y, Sasaki T. Okonogi T, et al. Nat Commun. 2024 Jan 9;15(1):183. doi: 10.1038/s41467-023-44205-y. Nat Commun. 2024. PMID: 38195621 Free PMC article. - Reward-related dynamical coupling between basolateral amygdala and nucleus accumbens.
Hsu CC, Madsen TE, O'Gorman E, Gourley SL, Rainnie DG. Hsu CC, et al. Brain Struct Funct. 2020 Jul;225(6):1873-1888. doi: 10.1007/s00429-020-02099-2. Epub 2020 Jun 18. Brain Struct Funct. 2020. PMID: 32556583 Free PMC article. - Minocycline Ameliorates Chronic Unpredictable Mild Stress-Induced Neuroinflammation and Abnormal mPFC-HIPP Oscillations in Mice.
Tabassum S, Misrani A, Huo Q, Ahmed A, Long C, Yang L. Tabassum S, et al. Mol Neurobiol. 2022 Nov;59(11):6874-6895. doi: 10.1007/s12035-022-03018-8. Epub 2022 Sep 1. Mol Neurobiol. 2022. PMID: 36048340
References
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. - PubMed
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. - PubMed
- Burwell RD, Witter MP. Basic anatomy of the parahippocampal region in monkeys and rats. Oxford University Press; New York: 2002.
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH081958/MH/NIMH NIH HHS/United States
- K08 MH069823-05/MH/NIMH NIH HHS/United States
- K08 MH069823/MH/NIMH NIH HHS/United States
- R01 MH081968/MH/NIMH NIH HHS/United States
- K08 MH098623/MH/NIMH NIH HHS/United States
- R01 MH081968-02/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases