Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism - PubMed (original) (raw)
Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism
Ann C Zovein et al. Dev Cell. 2010.
Abstract
Maintenance of single-layered endothelium, squamous endothelial cell shape, and formation of a patent vascular lumen all require defined endothelial cell polarity. Loss of beta1 integrin (Itgb1) in nascent endothelium leads to disruption of arterial endothelial cell polarity and lumen formation. The loss of polarity is manifested as cuboidal-shaped endothelial cells with dysregulated levels and mislocalization of normally polarized cell-cell adhesion molecules, as well as decreased expression of the polarity gene Par3 (pard3). beta1 integrin and Par3 are both localized to the endothelial layer, with preferential expression of Par3 in arterial endothelium. Luminal occlusion is also exclusively noted in arteries, and is partially rescued by replacement of Par3 protein in beta1-deficient vessels. Combined, our findings demonstrate that beta1 integrin functions upstream of Par3 as part of a molecular cascade required for endothelial cell polarity and lumen formation.
(c) 2010 Elsevier Inc. All rights reserved.
Figures
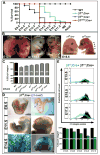
Figure 1
Loss of β1 integrin in the endothelium results in gene-dose dependant lethality. (A) VE-cadherin Cre deletion of β1 integrin gene in a heterozygous null background (β1f/n; Cre+, blue) results in mid-gestational lethality, as compared to late fetal lethality in homozygous floxed β1 integrin (β1f/f; Cre+, green). In contrast, VE-cadherin Cre mediated recombination of exon 3 floxed alleles leads to a sharp lethality at E13.5 (β1e3/e3; Cre+, red). (B) All deletions result in hemorrhaging (arrows) and edema (arrowheads) as evidenced in whole mount embryos. (C) Various β1 integrin deletion lines were evaluated at E12.5 for percentage of endothelial cells (ECs) that expressed β1 integrin via FACS analysis. Percentages are depicted in parenthesis. Note the mouse lines with earlier lethality have a greater reduction in β1, and the heterozygous β1 null (β1+/-) has a background level of 91%. (D) Genetic deletion of the β1f/f; Cre+ line, as demonstrated by β1 promoter driven LacZ expression, shows increasing endothelial deletion with advancing gestational age. (E) Endothelial cell β1 integrin protein expression, as measured by FACS, depicts a lag in protein loss as compared to genetic loss in D. Bottom graph depicts % of endothelial population with β1 integrin protein expression by FACS (β1f/f; Cre+ in green, β1f/+; Cre+ in black). (A-E) All graphical data shown as mean +/- SEM with a minimum of n=3 per data set, exception in (E) as data sets of n=2 do not depict error bars (E14.5 and E17.5). See also Figure S1.
Figure 2
β1 integrin endothelial deletion results in luminal occlusion, cuboidal cell shape, and stratification of the endothelial layer. (A) β-gal staining identifies β1 deleted endothelial cells (top panel) surrounding a nearly occluded lumen (arrow) in the E14.5 homozygous floxed (β1f/f; Cre+) animal. PECAM-1 (bottom, in black) also demonstrates an occluded endothelial lumen (arrow) at E15.5. Note that vessels appear occluded by endothelial cells (PECAM-1+), as also demonstrated in B (arrows). Panels are histological sections of skin with either β-gal (blue) or PECAM-1 (black), and nuclear stain in red. (B) Occlusion of the lumen was demonstrated in semithin sections of E15.5 β1f/f;Cre+ mice (arrows, top panel), and again with PECAM-1 expression (arrows, PECAM-1 in red). Endothelial deletion of β1 integrin also leads to abnormalities of smooth muscle cell (SMC) organization. While SMC morphology appears near normal in the semithin sections (arrowheads), α smooth muscle actin staining (SMA, green) depicts disorganization within SMC layers (arrowheads). (C) To ensure the occluded lumens were not a function of vascular constriction, muscle relaxants were administered to pregnant dams prior to sacrifice, and sections demonstrate no change in the occlusion phenotype (arrow). PECAM-1 in black, nuclear stain in red. (D) β1 integrin loss is associated with atypical cuboidal shape (black arrows) and stratification (brackets, and white arrows) in contrast to the normally flattened appearance of other adjacent endothelial cells (arrowheads). Top panels: E12.5 β1f/n;Cre+ endocardial cells exhibit β1-LacZ expression (β-gal staining in blue) after Cre mediated excision (counter stain in red or hemotoxylin-eosin staining in purple). β1 integrin ablated embryos also exhibit single layered squamous shaped endothelial cells (arrowheads) within the same section of cuboidal ECs or stratification, suggesting that full deletion does not occur within the entire endothelial population simultaneously. Lower panel: Stratification is also seen in E16.5 β1f/f;Cre+ (arrows) large vessel endothelium (PECAM-1 in red, SMA in green). (E) Altered cell shape at the ultrastructural level (arrows) is seen at E15.5 (β1f/f;Cre+), alongside normally shaped endothelial cells comprising the vessel wall (arrowheads). (A-E) Scale bars as labeled for each row. See also Figure S2.
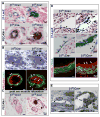
Figure 3
Luminal occlusion and loss of polarity is an arterial phenomenon. (A) Microarray analysis of endothelial cells (ECs), sorted based on β1 integrin protein expression (from E16.5 β1f/f;Cre+ embryos), exhibited increased levels of adhesion related genes, and a decrease in the polarity gene Par3. (B) Loss of β1 integrin protein (green) results in mis-localization of PECAM-1 (red) from laterally placed cell-cell contacts to global cell surface expression (arrows). TOPRO-3 nuclear stain in blue. (C) To confirm loss of β1 integrin and Par3 on a protein level, ECs sorted in the same manner were evaluated by Western blot. Sorted β1+ and β1- endothelial cells from β1f/f;Cre+ mice, and β1+ ECs from β1f/+;Cre+ mice were loaded equally by cell number, and demonstrated loss of β1 and Par3 protein (α-enolase loading control). (D) Normally polarized expression of VE-cadherin (top panel in red, SMA in green) at lateral cell-cell contacts (arrows) is dispersed and circumferentially expressed in cells occluding the lumen within β1f/f; Cre+ vessels (arrows). CD99 (bottom panels, green) demonstrates a polarized apical expression (arrowheads), and lateral co-localization with PECAM-1 (yellow, arrows), that after β1 integrin ablation redistributes to surround the cell at E15.5 (arrows), much like PECAM-1 (in red). (E) Luminal occlusion is distinctly noted in arteries (arrows), as delineated by PECAM-1 (top panels in black, bottom panels in red) and lack of EphB4 (bottom panels, green) expression. “A” denotes arteries, “V” veins, and “L” lymphatics. (F) The extent of occlusion can vary in mid-sized arteries (arrows) at E15.5 after endothelial β1 integrin deletion, but as compared to nearby veins is a distinctly arterial phenomenon. Lower panels are high magnification of vessels in upper panels. PECAM-1 in black. (G) Vessels were evaluated at E15.5, quantified for luminal patency, and confirmed that the phenotype is exclusively arterial (n=5 each, *p< .001). (H) Par3 protein expression is preferentially expressed in embryonic arteries, as evidenced in β1f/f;Cre- at E15.5 (Par3 in red, β1 integrin in green, TOPRO-3 nuclear stain in blue). Panels (a) – (c) are higher magnification of A – V pair above. (H-a,b) Par3 co-localizes with β1 integrin (yellow) in the basal aspect of the arterial endothelial layer (arrows), but is also prominent in the surrounding smooth muscle cell layer (in red, arrowheads). (H-c) Veins also express Par3 in conjunction with β1 (yellow, arrows), but to a lesser extent than arterial vessels. (I) To evaluate Par3 expression in vessel subtypes, dorsal aortas (A) and inferior vena cavas (V) of 4 week old and adult animals were flushed with Laemmli buffer and evaluated by Western blot. Primary human endothelial cells were evaluated for Par3 expression among vessel subtypes (human aortic endothelial cells – HAECs, human umbilical vein ECs - HUVECs, and human saphenous vein ECs – HSVECs). GAPDH and α-enolase loading controls. (J) Normal basal expression of Par3 (red) with co-expression of β1 integrin (in green, co-localization in yellow) in β1f/f; Cre- vessels (arrows) is aggregated and mis-localized in β1f/f; Cre+ vessels (arrows), with complete absence in β1 deleted cells within the vessel lumen (arrowheads). TOPRO-3 nuclear stain in blue. (B, D-F, H, J) Scale bars as labeled for each row. See also Figure S3.
Figure 4
Postnatal retinal β1 integrin deletion results in Par3 loss and abnormal endothelial cell polarity. (A) Wild-type (WT) retinal endothelial cell protein evaluated by Western blot demonstrates Par3 levels peak at P6-P9. (B) Par3 (red) is expressed in WT retinal vessels (P10) with β1 integrin (green, arrows). TOPRO-3 nuclear stain in blue. (C) Postnatal β1 integrin ablation induced by tamoxifen injection (from P2 to P7) in the β1f/n; iCre+ retina, results in large cyst-like outgrowths from the vasculature at P9 (arrows in right panel). Isolectin B4 (IsoB4) in red. (D) Postnatal tamoxifen induction when traced using a LacZ R26R Cre reporter line (R26R; iCre+) labels a small subset of retinal endothelia at P7, as compared to constitutive expression (R26R; Cre+). Arteries (A) and veins (V) labeled respectively. (E) After postnatal induction retinal endothelial cells were isolated (at P9) and evaluated by Western blot. β1 integrin protein is notably decreased in β1f/n; iCre+, as is Par3 protein levels (right column). (F) When β1f/n; iCre+ crossed to a EYFP R26R reporter undergoes β1 ablation, the β1 deleted retinal ECs (EYFP+ in green, arrows) become abnormally located in the vasculature in cyst-like structures (labeled by IsoB4 in red) and demonstrate loss of β1 protein (blue). (B-D, F) Scale bars as included for each row. See also Figure S4.
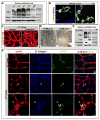
Figure 5
β1 integrin antibody blockade recapitulates the genetic deletion phenotype. (A) β1 integrin pharmacological blockade at P7 and evaluation at P10 demonstrates increased endothelial cell (EC) aggregation and cysts (arrows) with increased branching and decreased borders between arteries (A) and veins (V). Note the normally avascular area surrounding the retinal arteries is restricted after β1 antibody blockade (arrowheads). Isolectin B4 (IsoB4) in red. (B) Top panel: The cysts are comprised of endothelial cells as demonstrated by IsoB4 and TOPRO-3 (blue) nuclear staining (arrows). Bottom panels: When retinas are evaluated for pericyte coverage (NG2, green), there is no observed effect after β1 blockade, however ECs that are abnormally positioned with respect to the vasculature, or in cysts, are not covered by pericytes (arrows). PECAM-1 staining (brown) of histological sections demonstrates abnormal vessel morphology (arrows), and luminal occlusion (inset). (C) Dll4, an arterial marker (in green) was examined to evaluate whether arterial identity was affected as a result of β1 antibody blockade. While Dll4 expression was preserved (arrowheads), increased branching with closer proximity to the main arterial vessel is apparent (arrows, brackets). (D) To evaluate the effects of β1 blockade at different ages, retinas were injected either at P4, or in the adult, and evaluated 72hrs later. Left: The P4 blockade results in abnormal vascular patterning (IsoB4 in red) with excessive pericyte coverage (NG2 in green), and luminal occlusion (PECAM-1 in brown, arrows). Right: In the adult, very few abnormalities were encountered. Rarely small cysts could be seen (IsoB4 in red, inset), as well as modest thickening of the endothelial layer (PECAM-1 in brown, arrow), while pericyte coverage was normal. TOPRO-3 nuclear stain in blue. (A-D) Scale bars as shown for each row. See also Figure S5.
Figure 6
Par3 partially rescues lumen occlusion and cyst formation in endothelial β1 integrin ablation. (A-F) Postnatal animals (β1f/n; iCre+) were induced with tamoxifen (P2-P7) and retinal vasculature evaluated from P9-12. A subset was then rescued with Par3 lentiviral ocular delivery 48hrs prior to evaluation. (A) The large cysts observed with postnatal β1 integrin ablation were resolved significantly with lentiviral Par3 replacement, but a few endothelial cells (ECs) still displayed smaller atypical aggregates (arrows). Isolectin B4 (IsoB4) in red. (B) When β1f/n; iCre+ crossed to EYFP R26R (in green) retinas were rescued with FLAG-tagged Par3 lentivirus (blue), a rescued vessel demonstrates a patent lumen (arrowheads). In contrast, ECs that underwent β1 deletion (green) but were not rescued remain abnormally shaped with an occluded lumen (arrows). Asterisk denotes EYFP+ red blood cell. (C) On analysis of retinal semithin sections, β1 ablation resulted in vessel occlusion (arrowheads) that was partially rescued with Par3 protein (arrows). Boxed areas are magnified on right. (D) Retinal vessels were quantified for percentage of deletion (left) by assessing β-gal positive endothelial cells within the abdominal muscle - tamoxifen group (dark grey), tamoxifen + Par3 rescue group (light grey). The number of occluded vessels was quantified and compared to percent deletion in a ratio (right). The β1f/n; iCre+ tamoxifen group (dark grey) demonstrates that the occlusion phenotype is in direct proportion to the amount of deletion. There is a significant (*) decrease in the ratio of occluded vessels (%) to percent deletion with Par3 rescue (light grey). Data shown as mean +/- SEM, n=7 each group, p value = 0.025. (E) On a per animal basis, the percentage of deletion varies (circles), but averages at approximately 30% for both groups; while the percentage of occlusion (triangles) is dramatically reduced from 27% in the non-rescue to 16% in the rescued group. (F) Higher magnification depicting the vessel occlusion (or patency in the rescue) that was quantified in the retina (arrow). (A-C, F) Scale bars as labeled for each row. See also Figure S6.
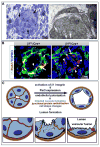
Figure 7
Arrest of lumen formation and excess of cytosolic vacuoles in β1 deleted endothelial cells (ECs). (A) Semithin (left) and EM (right) analysis of luminal occlusion in E15.5 β1f/f;Cre+ animals demonstrates accumulation of multiple vesicles/vacuoles within the cell cytoplasm (arrows). (B) Evaluation of Rab7 (red) in context of β1 integrin (green) demonstrates Rab7 in the basal aspect of the endothelium and at the smooth muscle cell (SMC)/ EC junction with β1 integrin (yellow), but minimal expression within the endothelial layer (arrowhead). Upon β1 deletion, a dramatic increase of Rab7 expression in β1 ablated ECs was noted (right panel, arrowheads) with some maintenance of co-expression with β1 integrin in the SMC layer (arrow). (C) Schema depicts a working model of the cascade of events that drive lumen formation. Activation of β1 integrin instructs Par3 expression which then allows for polarization of the endothelium. Vesicular fusion to the apical cell membrane results in redistribution of junctional and adhesion proteins (in red, arrows), change in cell shape from cuboidal to squamous, and acquisition of a vascular lumen.
Similar articles
- Par3/integrin β1 regulates embryo adhesion via changing endometrial luminal epithelium polarity†.
Peng J, Li X, Zhang Y, Hu J, Shang Y, Yin Y, Xiao Z. Peng J, et al. Biol Reprod. 2021 Jun 4;104(6):1228-1238. doi: 10.1093/biolre/ioab033. Biol Reprod. 2021. PMID: 33675651 - Requirement of β1 integrin for endothelium-dependent vasodilation and collateral formation in hindlimb ischemia.
Henning C, Branopolski A, Schuler D, Dimitroulis D, Huelsemann P, Nicolaus C, Sansone R, Ludolf Postma J, Eberhard D, Le Noble F, Kelm M, Lammert E, Heiss C. Henning C, et al. Sci Rep. 2019 Nov 15;9(1):16931. doi: 10.1038/s41598-019-53137-x. Sci Rep. 2019. PMID: 31729436 Free PMC article. - Rassf5 and Ndr kinases regulate neuronal polarity through Par3 phosphorylation in a novel pathway.
Yang R, Kong E, Jin J, Hergovich A, Püschel AW. Yang R, et al. J Cell Sci. 2014 Aug 15;127(Pt 16):3463-76. doi: 10.1242/jcs.146696. Epub 2014 Jun 13. J Cell Sci. 2014. PMID: 24928906 Free PMC article. - Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices.
Sacharidou A, Stratman AN, Davis GE. Sacharidou A, et al. Cells Tissues Organs. 2012;195(1-2):122-43. doi: 10.1159/000331410. Epub 2011 Oct 13. Cells Tissues Organs. 2012. PMID: 21997121 Free PMC article. Review. - Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices.
Davis GE, Koh W, Stratman AN. Davis GE, et al. Birth Defects Res C Embryo Today. 2007 Dec;81(4):270-85. doi: 10.1002/bdrc.20107. Birth Defects Res C Embryo Today. 2007. PMID: 18228260 Review.
Cited by
- Sarcoma Cells Secrete Hypoxia-Modified Collagen VI to Weaken the Lung Endothelial Barrier and Promote Metastasis.
Liu Y, Murazzi I, Fuller AM, Pan H, Irizarry-Negron VM, Devine A, Katti R, Skuli N, Ciotti GE, Pak K, Pack MA, Simon MC, Weber K, Cooper K, Eisinger-Mathason TSK. Liu Y, et al. Cancer Res. 2024 Apr 1;84(7):977-993. doi: 10.1158/0008-5472.CAN-23-0910. Cancer Res. 2024. PMID: 38335278 Free PMC article. - PDGFRα: Expression and Function during Mitral Valve Morphogenesis.
Moore K, Fulmer D, Guo L, Koren N, Glover J, Moore R, Gensemer C, Beck T, Morningstar J, Stairley R, Norris RA. Moore K, et al. J Cardiovasc Dev Dis. 2021 Mar 13;8(3):28. doi: 10.3390/jcdd8030028. J Cardiovasc Dev Dis. 2021. PMID: 33805717 Free PMC article. - A VE-cadherin-PAR3-α-catenin complex regulates the Golgi localization and activity of cytosolic phospholipase A(2)α in endothelial cells.
Odell AF, Hollstein M, Ponnambalam S, Walker JH. Odell AF, et al. Mol Biol Cell. 2012 May;23(9):1783-96. doi: 10.1091/mbc.E11-08-0694. Epub 2012 Mar 7. Mol Biol Cell. 2012. PMID: 22398721 Free PMC article. - Polarity in mammalian epithelial morphogenesis.
Roignot J, Peng X, Mostov K. Roignot J, et al. Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2):a013789. doi: 10.1101/cshperspect.a013789. Cold Spring Harb Perspect Biol. 2013. PMID: 23378592 Free PMC article. Review. - Endothelial Cords Promote Tumor Initial Growth prior to Vascular Function through a Paracrine Mechanism.
Zhao C, Zhang W, Zhao Y, Yang Y, Luo H, Ji G, Dong E, Deng H, Lin S, Wei Y, Yang H. Zhao C, et al. Sci Rep. 2016 Jan 14;6:19404. doi: 10.1038/srep19404. Sci Rep. 2016. PMID: 26762853 Free PMC article.
References
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. - PubMed
- Davis GE, Koh W, Stratman AN. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today. 2007;81:270–285. - PubMed
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. American journal of physiology. 2008;295:C545–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA126935/CA/NCI NIH HHS/United States
- HL085618/HL/NHLBI NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- K12-HD00850/HD/NICHD NIH HHS/United States
- CA126935/CA/NCI NIH HHS/United States
- R01 HL085618/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous