Emx2 and early hair cell development in the mouse inner ear - PubMed (original) (raw)
Emx2 and early hair cell development in the mouse inner ear
Matthew Holley et al. Dev Biol. 2010.
Abstract
Emx2 is a homeodomain protein that plays a critical role in inner ear development. Homozygous null mice die at birth with a range of defects in the CNS, renal system and skeleton. The cochlea is shorter than normal with about 60% fewer auditory hair cells. It appears to lack outer hair cells and some supporting cells are either absent or fail to differentiate. Many of the hair cells differentiate in pairs and although their hair bundles develop normally their planar cell polarity is compromised. Measurements of cell polarity suggest that classic planar cell polarity molecules are not directly influenced by Emx2 and that polarity is compromised by developmental defects in the sensory precursor population or by defects in epithelial cues for cell alignment. Planar cell polarity is normal in the vestibular epithelia although polarity reversal across the striola is absent in both the utricular and saccular maculae. In contrast, cochlear hair cell polarity is disorganized. The expression domain for Bmp4 is expanded and Fgfr1 and Prox1 are expressed in fewer cells in the cochlear sensory epithelium of Emx2 null mice. We conclude that Emx2 regulates early developmental events that balance cell proliferation and differentiation in the sensory precursor population.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures
Fig. 1
Expression of Emx2 in the inner ear. A) Little or no expression was detected in the otocyst at E10.5. B) In the cochlear duct at E12.5 the lateral, sensory region of the cochlear epithelium was labeled (between arrowheads) and there was detectable label in the roof of the duct (arrow). No expression was observed in the spiral ganglion (sg). C) At E14.5 the expression pattern in the cochlear duct was similar to that at E12.5. The image includes sections through 3 parts of the duct from the apex (a) of the spiral to the base (b). D–F) At E14.5 there was no expression in the anterior (D), lateral (E) or posterior (F) cristae. G–H) At E14.5 Emx2 was expressed in the saccular (G) and utricular (H) maculae (defined by arrowheads). I) At E14.5 Emx2 was expressed in the endolymphatic duct (ed). Scale bar = 100 μm.
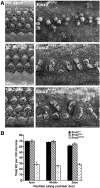
Fig. 2
Whole dissected cochlear spirals from normal and null neonatal mice. In Emx2 KO/KO pups the cochlear duct was about half a turn shorter with a mean length of about 5.8 mm as opposed to 7.3–7.5 mm. There was no significant difference between Emx2 +/+ and Emx2 KO/+ animals at E18.5 but in Emx2 KO/KO animals the cochlea was significantly shorter (p < 0.001; the numbers of ears/embryos/litters were 8/6/3 for Emx2 +/+, 31/21/6 for Emx2 KO/+ and 23/15/6 for Emx2 KO/KO).
Fig. 3
A) Scanning electron micrographs from apical, middle and basal regions of Emx2 +/+ and Emx2 KO/KO neonatal mice. The bottom left panel shows 4 rows of hair cells running from left to right of the image. The 3 upper rows are composed of outer hair cells and the bottom row is composed of inner hair cells. One of the outer hair cells is marked by 2 asterisks, between which lies the V-shaped hair bundle composed of stereocilia. The cell surface inside the V is covered with microvilli whereas outside the V it is smooth. The hair bundles on the inner hair cells have a less pronounced V shape. Note that in Emx2 KO/KO mice there were fewer hair cells in less defined rows. Most hair cells existed singly or in pairs and lacked elements of their normal planar polarity. In morphological terms they appeared to differentiate normally if slightly slower than normal. Signs of delayed hair cell differentiation were most obvious in the apex at this stage. Scale bar = 20 μm. B) Counts of the numbers of hair cells per 100 μm of epithelium revealed no difference between Emx2 +/+ and Emx2 KO/+ pups but a decrease of about 55% in the Emx2 KO/KO pups (_t_-test — p < 0.001 between Emx2 KO/KO and both Emx2 KO/+ and Emx2 +/+ mice for all parts of the cochlea).
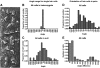
Fig. 4
A) Scanning electron micrograph from an Emx2 KO/KO neonatal pup to illustrate measurements of cell orientation. Orientation was measured as the angle between the line of the hair cell bundle (hc) and the long axis of the epithelium (e). A cell with normal orientation would be at 0° to the epithelial axis. The shape of the hair bundle was not always well defined but cell orientation was further defined by the presence of microvilli on one side (arrow 1). No measurement was greater than ± 180°. Many hair cells existed in pairs facing either the same (arrow 1) or less frequently the opposite direction (arrow 2). Some cells existed in groups of 3 (*) or more. B) Range of angles for 517 hair cells recorded from apical, middle and basal regions of the cochlea in 4 different Emx2 KO/+ pups. C) Range of angles for 1172 hair cells recorded from apical, middle and basal regions of the cochlea in 4 different Emx2 KO/KO pups. Despite the apparent lack of alignment most cells were orientated within a range of 180° towards one side of the epithelium. D) Range of angles for individual hair cells in pairs in Emx2 KO/KO pups. The distribution was similar to that of single cells. E) Range of angles between cells within pairs. Note the bimodal distribution.
Fig. 5
Transmission electron micrographs of sections through hair cells in Emx2 +/+ and Emx2 KO/KO neonatal pups. A) In Emx2 +/+ animals the hair cells were separated by supporting cells. B) In Emx2 KO/KO pups the hair cells were not spaced evenly within the sensory epithelium and were often very close to their neighbours. C) In Emx2 KO/KO pups some hair cells made direct contact with each other in both apical and basal regions of the cell (arrows). Part of a supporting cell is indicated by an asterisk. hc — hair cells, sc — supporting cells, hb — hair bundle. Scale bar = 5 µm.
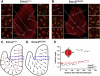
Fig. 6
A) A surface view of the saccular macula in an Emx2 +/+ mouse at E18.5. The central panel shows the whole epithelium with the actin labeled with rhodamine-conjugated phalloidin. The white boxes indicate adjacent panels at higher magnification. In these panels the hair cell surfaces, including the hair bundles, appear red with the kinocilia labeled with antibodies to β-tubulin in green. The dotted arrows indicate planar cell polarity with the kinocilium at the leading edge. The striola defines the line about which planar polarity is reversed (see panel C). In supporting cells the phalloidin only labeled the apical perimeter of the cells. Scale bar = 100 µm (insets = 10 µm). B) The saccular macula was smaller in Emx2 KO/KO at E18.5. Hair cells had differentiated hair bundles with kinocilia and stereociliar bundles. Closer examination showed that reversal of cell polarity across the striola was absent and that all hair cells faced the outer margin of the macula, as indicated by the dotted arrows in the smaller panels. Scale bar = 100 µm (insets = 10 µm). C) Diagram of hair cell orientation in an Emx2 +/+ mouse at E18.5. The striolar boundary is shown by a dotted red line. Note that the inner edge of the horseshoe shaped epithelium is the medial side. D) Diagram of hair cell orientation in an Emx2 KO/KO at E18.5. The striolar boundary as defined by the reversal in cell polarity was absent. E) Measurements of the planar orientation of hair cells from analysis of the boxed regions in C and D show that cell polarity reversal was missing in Emx2 KO/KO mice at E18.5 even though the cells were aligned within a range of no more than 30° to the epithelial axis. Red triangles represent measurements from Emx2 KO/KO pups and black squares from Emx2 +/+ pups. Note the change in polarity at the polarity reversal line about 140 μm from the lateral border only in the Emx2+/+ pups.
Fig. 7
Scanning electron microscope images of hair cells from across the striola in the utricular macula at E18.5. Each image shows 2–3 hair bundles each composed of a single, long kinocilium (k) and a bundle of stereocilia (s). The stereocilia are graded in length and diameter, with the shortest ones located on the opposite side of the cell to the kinocilium. Thus cell polarity is readily assigned (large arrows). In Emx2 +/+ mice the polarity is reversed above and below the striola and the boundary at the striola is clear (*). In Emx2 KO/KO mice the boundary as defined by reversal of polarity is absent (**) but polarity is uniform across the entire utricular epithelium. Scale bar = 10 µm.
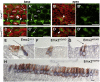
Fig. 8
Markers for inner and outer hair cells in Emx2 +/+ and Emx2 KO/KO mice at E18.5. A) Immunolabel for GFAP (green) in the basal region of the cochlea from Emx2 +/+ mice at E18.5. Actin is labeled in red with phalloidin. The apical regions of the inner pillar cells (single arrowhead), Deiter's cells (dc) and inner hair cells (ihc and arrow) were labeled for GFAP but outer hair cells (ohc) were not. An outer pillar cell is indicated by the double arrowhead. B) As in panel A but from the apical region of the cochlea. Some cells, probably undifferentiated hair cells and supporting cells, were labeled for GFAP (arrowhead). C) As in panel A but from Emx2 KO/KO pups. The hair cells (arrow) were labeled but there was little sign of labeling in Deiter's or pillar cells. D) As in panel C but from the apical end of the cochlea. E) A section through the neonatal cochlea of an Emx2 +/+ pup. All hair cells were labeled with antibody to myosin VIIa (brown) but only the inner hair cells (arrow) were labeled by in situ hybridization for Fgf8 (blue nucleus). F) The two remaining rows of hair cells in the Emx2 KO/KO pups labeled for both myosin VIIa and for Fgf8. G) An occasional hair cell was negative for Fgf8 but retained the morphology of a normal inner hair cell (arrow). H) In a section along the organ of Corti of an Emx2 KO/KO pup nearly all hair cells labeled for Fgf8. Scale bars = 10 µm.
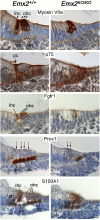
Fig. 9
Antibody labeling for markers of hair cells and supporting cells in the organ of Corti at stage E16.5 (Myosin VIIa, p75, Fgfr1) and E18.5 (Prox1, S100A). Myosin VIIa revealed the single row of inner hair cells (ihc) and 3 rows of outer hair cells (ohc) in Emx2 +/+ animals but only 2 rows of hair cells in Emx2 KO/KO animals. Pillar cells (pc), normally strongly labeled with antibodies to p75, appeared to be absent in Emx2 KO/KO pups. Expression of Fgfr1 was much lower in the Emx2 KO/KO pups, both in supporting cells and remaining hair cells. Expression of Prox1 was also much lower in Emx2 KO/KO pups. It was expressed only in supporting cells, specifically pillar cells and Deiter's cells (dc) at this stage, the hair cells being indicated by arrows. Antibodies to S100A labeled inner hair cells and inner phalangeal cells (iph) in Emx2 +/+ mice but labeled the outer hair cells less strongly. In Emx2 KO/KO pups the boundary between inner and outer hair cells, defined by the pillar cells, was absent but the remaining hair cells were labeled strongly. Scale bar = 30 μm.
Similar articles
- Emx2 lineage tracing reveals antecedent patterns of planar polarity in the mouse inner ear.
Goodrich EJ, Deans MR. Goodrich EJ, et al. Development. 2024 May 15;151(10):dev202425. doi: 10.1242/dev.202425. Epub 2024 May 16. Development. 2024. PMID: 38682291 Free PMC article. - Prox1 interacts with Atoh1 and Gfi1, and regulates cellular differentiation in the inner ear sensory epithelia.
Kirjavainen A, Sulg M, Heyd F, Alitalo K, Ylä-Herttuala S, Möröy T, Petrova TV, Pirvola U. Kirjavainen A, et al. Dev Biol. 2008 Oct 1;322(1):33-45. doi: 10.1016/j.ydbio.2008.07.004. Epub 2008 Jul 9. Dev Biol. 2008. PMID: 18652815 - Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear.
Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Zine A, et al. J Neurosci. 2001 Jul 1;21(13):4712-20. doi: 10.1523/JNEUROSCI.21-13-04712.2001. J Neurosci. 2001. PMID: 11425898 Free PMC article. - A balance of form and function: planar polarity and development of the vestibular maculae.
Deans MR. Deans MR. Semin Cell Dev Biol. 2013 May;24(5):490-8. doi: 10.1016/j.semcdb.2013.03.001. Epub 2013 Mar 15. Semin Cell Dev Biol. 2013. PMID: 23507521 Free PMC article. Review. - Atoh1 and other related key regulators in the development of auditory sensory epithelium in the mammalian inner ear: function and interplay.
Zhong C, Fu Y, Pan W, Yu J, Wang J. Zhong C, et al. Dev Biol. 2019 Feb 15;446(2):133-141. doi: 10.1016/j.ydbio.2018.12.025. Epub 2018 Dec 31. Dev Biol. 2019. PMID: 30605626 Review.
Cited by
- Contributions of mirror-image hair cell orientation to mouse otolith organ and zebrafish neuromast function.
Ono K, Jarysta A, Hughes NC, Jukic A, Chang HHV, Deans MR, Eatock RA, Cullen KE, Kindt K, Tarchini B. Ono K, et al. bioRxiv [Preprint]. 2024 Sep 6:2024.03.26.586740. doi: 10.1101/2024.03.26.586740. bioRxiv. 2024. PMID: 39282410 Free PMC article. Preprint. - A cochlear progenitor pool influences patterning of the mammalian sensory epithelium via MYBL2.
Young CA, Burt E, Munnamalai V. Young CA, et al. Development. 2024 Sep 1;151(17):dev202635. doi: 10.1242/dev.202635. Epub 2024 Sep 10. Development. 2024. PMID: 39254648 Free PMC article. - Emx2 lineage tracing reveals antecedent patterns of planar polarity in the mouse inner ear.
Goodrich EJ, Deans MR. Goodrich EJ, et al. Development. 2024 May 15;151(10):dev202425. doi: 10.1242/dev.202425. Epub 2024 May 16. Development. 2024. PMID: 38682291 Free PMC article. - Region-specific reversal of epidermal planar polarity in the rosette fancy mouse.
Cetera M, Sharan R, Hayward-Lara G, Phillips B, Biswas A, Halley M, Beall E, vonHoldt B, Devenport D. Cetera M, et al. Development. 2023 Sep 1;150(17):dev202078. doi: 10.1242/dev.202078. Epub 2023 Sep 7. Development. 2023. PMID: 37622728 Free PMC article. - The dark kinase STK32A regulates hair cell planar polarity opposite of EMX2 in the developing mouse inner ear.
Jia S, Ratzan EM, Goodrich EJ, Abrar R, Heiland L, Tarchini B, Deans MR. Jia S, et al. Elife. 2023 May 5;12:e84910. doi: 10.7554/eLife.84910. Elife. 2023. PMID: 37144879 Free PMC article.
References
- Boncinelli E. Homeobox genes and disease. Curr. Opin. Genet. Dev. 1997;7:331–337. - PubMed
- Brown S.D., Hardisty-Hughes R.E., Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat. Rev. Genet. 2008;9:277–290. - PubMed
- Cecchi C. Emx2: a gene responsible for cortical development, regionalization and area specification. Gene. 2002;291:1–9. - PubMed
- Cecchi C., Boncinelli E. Emx homeogenes and mouse brain development. Trends Neurosci. 2000;23:347–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous