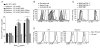Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84 - PubMed (original) (raw)
Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84
Jennifer L Cannons et al. Immunity. 2010.
Abstract
CD4(+) T cells deficient in signaling lymphocyte activation molecule (SLAM)-associated protein (SAP) exhibit a selective impairment in adhesion to antigen-presenting B cells but not dendritic cells (DCs), resulting in defective germinal center formation. However, the nature of this selective adhesion defect remained unclear. We found that whereas T cell:DC interactions were primarily integrin dependent, T cell:B cell interactions had both an early integrin-dependent phase and a sustained phase that also required SAP. We further found that the SLAM family member CD84 was required for prolonged T cell:B cell contact, optimal T follicular helper function, and germinal center formation in vivo. Moreover, both CD84 and another SLAM member, Ly108, mediated T cell adhesion and participated in stable T cell:B cell interactions in vitro. Our results reveal insight into the dynamic regulation of T cell:B cell interactions and identify SLAM family members as critical components of sustained T cell:B cell adhesion required for productive humoral immunity.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
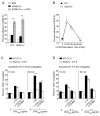
Figure 1. Integrin-mediated adhesion is critical for initial but not prolonged T:B cell conjugation
(A–B) Adhesion of pre-activated WT and Sh2d1a−/− CD4+ T cells to recombinant ICAM2-Fc following α-CD3 stimulation (A) 10 min. and (B) 15–60 min. Results presented as the mean percent adhesion (A) n=3 ± SEM. (B) n=6 ± SEM. (C–D) Conjugation efficiency after pre-incubation with blocking integrin antibodies then incubation for 10–30 min with OVA323 pulsed B cells (C), mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events (n=3) or (D) DCs, mean ± SEM frequency of CD4+CD11c+ conjugates in total CD4+ events (n=3). **p<0.005.
Figure 2. Importance of SAP in T:B adhesion is consistent with SLAM family member expression on activated B cells
(A) OT-II T:B cell conjugation assays were conducted using CD4+ T cells transiently transfected with DNA constructs expressing either GFP, GFP-SAP, GFP-SAP(R78A), or GFP-SAP(R55L), n=6, mean ± SEM frequency of CD4+CD19+ conjugates in total GFP+CD4+ events, **p<0.005 (See Figure S2 for western blot and representative FACS plot). (B) CD11c+ splenic DCs, splenic B cells and LPS-activated B cells were assessed for SLAM, Ly108, and CD84 expression (n=3). (C–D) Day 9 post-NP-OVA in alum immunization, (C) B cells or (D) Tfh (CD4+CD44+CXCR5hiPD-1hi) and non-Tfh (CD4+CD44+CXCR5loPD-1lo) cells were evaluated for Ly108 and CD84 expression (n=2, 2–5 mice/genotype).
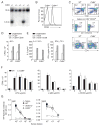
Figure 3. Targeted disruption of mouse Cd84 gene does not affect T and B cell development or in vitro stimulation
(A) Screening for homologous recombination by Southern blot of NcoI digested DNA. WT allele:10kb. Disrupted allele:6.5kb. Targeting vector and locus are shown in Figure S3A. (B) Surface CD84 expression on WT and Cd84−/− splenic B cells. (C) Top panel: thymocytes stained with α-CD4 and α-CD8. Middle panel: thymocytes stained with CD1d-αGalCer tetramers, α-CD4, α-CD24, and α–TCRβ to evaluate NKT cells. Bottom panel: splenocytes stained with α-CD19 and α-CD4 (n=3, 3 mice/genotype). (D) WT and Cd84−/− T cells were stimulated with α-CD3+/− α-CD28 and evaluated for proliferation and (E) IL-2 and IFN-γ production (n=3). (F) WT and Cd84−/− B cells were stimulated for 48 h with LPS, α-IgM, or α-CD40 and evaluated for proliferation (n=4). (G) WT, Sh2d1a−/−, and Cd84−/− mice were immunized with the type II T-independent antigen, NP-Ficoll, and assessed for NP-specific antibody production, day 21 (n=2, 5 mice/genotype).
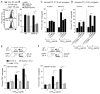
Figure 4. Impaired humoral response in the absence of CD84
WT, Sh2d1a−/−, and Cd84−/− mice were immunized with T-dependent antigen NP-OVA in alum or Ribi. (A) GC B cell development evaluated by gating on B220+IgDlo cells: GC B cells are FashiGL-7+. (B) Percentage of B cells with GC markers at day 4, 9 and 17 post-immunization. (C) Splenic GC were detected via staining with IgD and GL-7 day 9. (D) GC number/section, day 9. (E) GC size, day 9. * Size of the rare (2) GC identified in Sh2d1a−/− sections. (F) NP-specific IgG ASCs in the spleen, day 9. (G) Long-lived NP-specific plasma cell responses were measured in bone marrow, day 30. (H) NP-specific [NP-(30)], day 30 (See Figure S4A for high affinity NP-specific [NP-(3)] responses). Results are shown from mice immunized with NP-OVA in alum (A, B and H, n=2, 3–5 mice/genotype/time point) or NP-OVA in Ribi (C, D, E, F and G n=3, 6–8 mice/genotype/time point). Similar results were obtained with either adjuvant. (I) Cd84−/− or WT sorted naïve CD4+CD62L+CD44lo T cells and WT B cells were co-transferred into Rag2−/− hosts. Day 25 following reconstitution, Rag2−/− mice were immunized with SRBCs and evaluated for GC development (See Figure S4B for antibody titres), n=2, 4 mice/genotype/time point.
Figure 5. Evaluation of Tfh cells
(A–B) Flow cytometric analysis of cells expressing Tfh markers from WT, Sh2d1a−/−, and Cd84−/− mice at day 4 and 9 post-immunization with NP-OVA in alum. (A) Representative FACS plots (gated on CD4+ cells). (B) Percent Tfh cells, n=2, 2–5 mice/genotype/time point. (C–D) WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ T cells were transferred into Sh2d1a−/− hosts subsequently immunized with NP-OVA in alum (n=2, 3 mice/genotype/time point). (C) Number of WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ Tfh (CD4+CXCR5hiPD-1hi) cells day 4 and 7 post-immunization. (D) IL-21 production from WT, Sh2d1a−/−, or Cd84−/− OT-II GFP+ cells. (See Figure S5 for Tfh IL-21 production)
Figure 6. Cd84−/− OT-II T cells are defective in adhesion to cognate B cells
(A-B) Conjugation efficiency of pre-activated WT and Cd84−/− OT-II T cells with OVA323 pulsed splenic (A) CD11c+ DCs, mean ± SEM frequency of CD4+CD11c+ conjugates in total CD4+ events (n=4, representative FACS plots, right panel), (B) LPS-activated B cells, mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events (n=4, representative FACS plots, right panel) (*p<0.05, **p<0.005). (C) In vivo contact durations between T cells of indicated genotypes and MD4 B cells as measured by intravital microscopy between 60–72 h after immunization with HEL-OVA in alum. Individual contact durations (left) and their distribution (right) are shown. A total of 125, 120, and 49 contacts pooled from 3 experiments for WT, Cd84−/−, and Sh2d1a−/− T cells, respectively. Mean contact times: WT 14.5+/−1.2 min; Cd84−/− 8.5+/−2.1 min; Sh2d1a−/− 3.5+/−1.6 min. The p values were calculated by nonparametric one-way ANOVA. (D) Recovery of MD4 B cells from draining lymph nodes 96 h post-HEL-OVA immunization with or without exogenous OT-II T cells of indicated genotypes. Each symbol represents 1 of 4 mice/group. Data from 1 of 3 experiments with similar results are shown.
Figure 7. CD84 and Ly108 are adhesive receptors and contribute to long-lived stable T:B cell conjugates
(A) Ly108 expresssion on Tfh (CD4+CXCR5hiPD-1hi) cells from WT and Cd84−/− mice post-NP-OVA in alum immunization. (B) Evaluation of pre-activated WT and Sh2d1a−/− T cell adhesion to recombinant ICAM2-Fc, Ly108-Fc, and CD84-myc.HIS assessed for 30 min (n=4). (C-D) Conjugation efficiency of blasted WT OT-II T cells, pre-incubated with α-CD84 or α-Ly108, with OVA323-pulsed LPS-activated B cells (C), mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events (n=4) or DCs (D), mean ± SEM frequency of CD4+CD11c+ conjugates in total CD4+ events (n=3). (E–F) Conjugation frequencies of pre-activated WT, Sh2d1a−/−, and Cd84−/− OT-II T cells with OVA323-pulsed LPS-activated B cells for 30 min from (E) Sle.1b congenic or (F) Slamf6−/− mice, mean ± SEM frequency of CD4+CD19+ conjugates in total CD4+ events, n=3 (See Figure S6 for representative FACS plot). (*p<0.05, **p<0.005).
Similar articles
- Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150).
Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Yusuf I, et al. J Immunol. 2010 Jul 1;185(1):190-202. doi: 10.4049/jimmunol.0903505. Epub 2010 Jun 4. J Immunol. 2010. PMID: 20525889 Free PMC article. - SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase.
McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. McCausland MM, et al. J Immunol. 2007 Jan 15;178(2):817-28. doi: 10.4049/jimmunol.178.2.817. J Immunol. 2007. PMID: 17202343 - SAP-regulated T Cell-APC adhesion and ligation-dependent and -independent Ly108-CD3ζ interactions.
Chu C, Wang Y, Zhang X, Ni X, Cao J, Xu W, Dong Z, Yuan P, Wei W, Ma Y, Zhang L, Wu L, Qi H. Chu C, et al. J Immunol. 2014 Oct 15;193(8):3860-71. doi: 10.4049/jimmunol.1401660. Epub 2014 Sep 12. J Immunol. 2014. PMID: 25217164 - SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions.
Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. Detre C, et al. Semin Immunopathol. 2010 Jun;32(2):157-71. doi: 10.1007/s00281-009-0193-0. Epub 2010 Feb 10. Semin Immunopathol. 2010. PMID: 20146065 Free PMC article. Review. - Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules.
Ma CS, Nichols KE, Tangye SG. Ma CS, et al. Annu Rev Immunol. 2007;25:337-79. doi: 10.1146/annurev.immunol.25.022106.141651. Annu Rev Immunol. 2007. PMID: 17201683 Review.
Cited by
- Modulation of SAP dependent T:B cell interactions as a strategy to improve vaccination.
Hu J, Havenar-Daughton C, Crotty S. Hu J, et al. Curr Opin Virol. 2013 Jun;3(3):363-70. doi: 10.1016/j.coviro.2013.05.015. Epub 2013 Jun 3. Curr Opin Virol. 2013. PMID: 23743125 Free PMC article. Review. - The survival and function of IL-10-producing regulatory B cells are negatively controlled by SLAMF5.
Radomir L, Kramer MP, Perpinial M, Schottlender N, Rabani S, David K, Wiener A, Lewinsky H, Becker-Herman S, Aharoni R, Milo R, Mauri C, Shachar I. Radomir L, et al. Nat Commun. 2021 Mar 25;12(1):1893. doi: 10.1038/s41467-021-22230-z. Nat Commun. 2021. PMID: 33767202 Free PMC article. - Neonatally imprinted stromal cell subsets induce tolerogenic dendritic cells in mesenteric lymph nodes.
Pezoldt J, Pasztoi M, Zou M, Wiechers C, Beckstette M, Thierry GR, Vafadarnejad E, Floess S, Arampatzi P, Buettner M, Schweer J, Fleissner D, Vital M, Pieper DH, Basic M, Dersch P, Strowig T, Hornef M, Bleich A, Bode U, Pabst O, Bajénoff M, Saliba AE, Huehn J. Pezoldt J, et al. Nat Commun. 2018 Sep 25;9(1):3903. doi: 10.1038/s41467-018-06423-7. Nat Commun. 2018. PMID: 30254319 Free PMC article. - Harnessing the B Cell Response in Kidney Transplantation - Current State and Future Directions.
Anwar IJ, DeLaura IF, Gao Q, Ladowski J, Jackson AM, Kwun J, Knechtle SJ. Anwar IJ, et al. Front Immunol. 2022 Jun 9;13:903068. doi: 10.3389/fimmu.2022.903068. eCollection 2022. Front Immunol. 2022. PMID: 35757745 Free PMC article. Review.
References
- Al-Alwan MM, Rowden G, Lee TDG, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. - PubMed
- Barber DF, Long EO. Coexpression of CD58 or CD48 with intracellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J Immunol. 2003;170:294–299. - PubMed
- Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VLJ, Amigorena S. Requirements of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous