Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities - PubMed (original) (raw)
Review
Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities
Renata Santos et al. Antioxid Redox Signal. 2010.
Abstract
Mitochondrial dysfunction and oxidative damage are at the origin of numerous neurodegenerative diseases like Friedreich ataxia and Alzheimer and Parkinson diseases. Friedreich ataxia (FRDA) is the most common hereditary ataxia, with one individual affected in 50,000. This disease is characterized by progressive degeneration of the central and peripheral nervous systems, cardiomyopathy, and increased incidence of diabetes mellitus. FRDA is caused by a dynamic mutation, a GAA trinucleotide repeat expansion, in the first intron of the FXN gene. Fewer than 5% of the patients are heterozygous and carry point mutations in the other allele. The molecular consequences of the GAA triplet expansion is transcription silencing and reduced expression of the encoded mitochondrial protein, frataxin. The precise cellular role of frataxin is not known; however, it is clear now that several mitochondrial functions are not performed correctly in patient cells. The affected functions include respiration, iron-sulfur cluster assembly, iron homeostasis, and maintenance of the redox status. This review highlights the molecular mechanisms that underlie the disease phenotypes and the different hypothesis about the function of frataxin. In addition, we present an overview of the most recent therapeutic approaches for this severe disease that actually has no efficient treatment.
Figures
FIG. 1.
Diagnostic criteria for typical FRDA according to Harding (140).
FIG. 2.
Structure of the FXN gene and transcription maps. The structures of the FXN gene, except for the nontranslated exon 6, and of the three transcripts, are depicted. Transcript encoding isoform 2 results from an alternative splicing at exon 4′). Transcript encoding isoform 1a uses the exon 5b that is located 40 kb downstream of exon 5a. The GAA-repeat expansion in the first intron is indicated. The gray regions are not translated. Exons and introns are represented in different scales.
FIG. 3.
Amino acid sequence comparison of the three human frataxin isoforms. The mature forms are represented in bold, and the C-terminal variant region is boxed (isoform 1, 210 amino acids; isoform 1a, 171 amino acids; and isoform 2, 196 amino acids). Clustal W software was used for sequence alignment.
FIG. 4.
Comparison between the nucleotide sequences of the FXN Alu element and the _Alu_Sx consensus. The human FXN Alu element contains an expanded A5TACA5 sequence (A6TACA16, bold) followed by the GAA repeats (underlined). The most frequent number of GAA triplets (nine repeats) is represented. The flanking direct repeat is boxed.
FIG. 5.
Sequence and origin of the GAA triplet-repeat expansion in the FXN gene. (A) Number of GAA-triplet repeats in normal, borderline, and expanded alleles. The phenotypic consequence of alleles containing 35 to 43 repeats is not known. Borderline alleles can cause disease with mild phenotypes or not. (B) Model for the origin and evolution of GAA triplet-repeat expansion [see text for explanation; adapted from (209)].
FIG. 6.
Distribution of frataxin point mutations. (A) Mutations in the ATG codon are represented by the symbol φ; frameshifts, by the symbol Δ; and splice-site mutations by arrows. *Missense mutations and the amino acid changes. Regions encoding the mitochondrial addressing sequence are in gray, and those encoding the mature frataxin protein are in white. Most of missense mutations are distributed in the conserved exons 3-5a. (B) Distribution of missense mutations in the frataxin structure. Changes resulting in typical disease presentation are in red, changes resulting in atypical disease presentation are in blue, and those that can result in both typical and atypical disease presentation are in green. The YASARA View software was used to visualize the structure of the human frataxin deposited in Protein Data Bank (PDB ID: 1ekg). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
FIG. 6.
Distribution of frataxin point mutations. (A) Mutations in the ATG codon are represented by the symbol φ; frameshifts, by the symbol Δ; and splice-site mutations by arrows. *Missense mutations and the amino acid changes. Regions encoding the mitochondrial addressing sequence are in gray, and those encoding the mature frataxin protein are in white. Most of missense mutations are distributed in the conserved exons 3-5a. (B) Distribution of missense mutations in the frataxin structure. Changes resulting in typical disease presentation are in red, changes resulting in atypical disease presentation are in blue, and those that can result in both typical and atypical disease presentation are in green. The YASARA View software was used to visualize the structure of the human frataxin deposited in Protein Data Bank (PDB ID: 1ekg). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
FIG. 7.
Frataxin structure and homology. (A) Schematic representation of the structural elements found in human frataxin, as described at
www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/
(PDB: 1ekg). A color code identifies the primary structure conservation with cold colors, indicating low conservation, whereas hot colors indicate the most-conserved residues. (B) Surface charges distribution in human frataxin structure (PDB: 1ekg). Negative potential is represented in red, and positive potential is represented in blue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
FIG. 8.
Maturation of human and yeast frataxin by mitochondrial processing protease. Maturation of precursor (p) frataxin by MPP is a sequential two-step cleavage originating the intermediate (i) and the mature (m) forms. The human frataxin is synthesized as a 210-amino acid precursor, and processing in vitro may originate m56-FXN and m78-FXN, but only the m81-FXN mature form has a functional significance in vivo. For the yeast frataxin (174-amino acid precursor), only one mature form has been detected. It is interesting to note that frataxin proteins show a higher apparent molecular weight on SDS-PAGE gels than predicted because of the acidic nature of the N-terminal α-helix (279). The apparent sizes for FXN are described in (63, 279), and for Yfh1, are described in (38, 131).
FIG. 9.
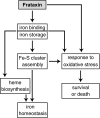
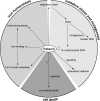
Hypothesis on the cellular functions of frataxin. Frataxin is an iron-binding protein involved in mitochondrial iron storage or iron use or both. These functions are important for maintenance of the overall cellular iron homeostasis and redox status. Frataxin deficiency causes impairment of Fe-S cluster and heme biosynthesis, oxidative stress, and cell death.
FIG. 10.
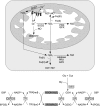
A model for Fe-S cluster protein assembly in human cells. In mitochondria and cytosol of mammalian cells, cysteine desulfurases (m-NFS1 and c-NFS1) remove sulfur from free cysteine and transfer it to the scaffold ISCU proteins (m-ISCU and c-ISCU). The proposed function of frataxin is to deliver iron to the desulfurase/scaffold complex for de novo biogenesis of [2Fe-2S] and [4Fe-4S] clusters in the mitochondria. This synthesis also requires the redox proteins ferrredoxin (FDX) and ferredoxin reductase (FDXR). The clusters are transiently bound to the scaffolds before being released and incorporated into recipient apoproteins. These steps are facilitated by the HSPA9 and HSCB chaperones in the mitochondria, and possibly by the NUBP1, NUBP2, NARF1, and CIAO1 chaperones in the cytosol. The cytosolic assembly of Fe-S clusters requires an unknown precursor exported from the mitochondria by the ABCB7 transporter.
FIG. 11.
Mapping of frataxin residues that interact with ferrochelatase. (A) FXN and Yfh1 amino acid sequence alignment showing the frataxin residues, identified by NMR spectroscopy, that show a chemical-shift perturbation as a result of complex formation with ferrochelatase (25, 143). (B) Residue visualization on the human frataxin structure by using the YASARA View software (PDB ID: 1ekg). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
FIG. 12.
ROS production and cellular antioxidant defense enzymes. Superoxide anion (O2•−) is produced by complexes I and III of the electron-transport chain and converted into hydrogen peroxide (H2O2) by superoxide dismutases (SOD) or into peroxynitrite (ONOO−) by reacting with nitric oxide (NO). H2O2 can react with ferrous iron to produce the hydroxyl radical (HO•). Glutathione (GSH/GSSG) is a tripeptide synthesized in two steps from glutamic acid, cysteine, and glycine. H2O2 and other peroxides are detoxified by glutathione peroxidases (GPXs), which oxidizes glutathione. GSSG is reduced to GSH by glutathione reductase (GR) by using electrons from NADPH. NADPH is regenerated by the pentose phosphate pathway enzymes, glucose 6-phosphate dehydrogenase (G6PDH), and 6-phosphogluconate dehydrogenase. Other enzymes that scavenge H2O2 and peroxides are catalases and peroxiredoxins (PRXs). Peroxiredoxins also can scavenge ONOO−. The cellular thiol redox status is maintained by the thioredoxin (TRX)/thioredoxin reductase (TR) and glutathione/glutaredoxin systems by reducing the oxidized sulfhydryl groups of proteins.
FIG. 13.
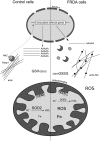
Frataxin deficiency leads to oxidative stress. In frataxin-deficient cells, the Nrf2-dependent Phase II antioxidant defense pathway is impaired. Actin is glutathionylated, and actin fibers are disorganized and not associated with Keap1 and Nrf2. Consequently, expression of the genes controlled by Nrf2, such as the mitochondrial SOD2, is not induced on treatment of frataxin-deficient cells with oxidants. The total glutathione concentration may be decreased, in addition to increase in the levels of the oxidized form (GSSG) and to more glutathione bound to proteins.
FIG. 14.
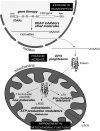
Therapeutic strategies for the treatment of FRDA. No efficient therapy is now available to treat patients. Given that the frataxin function is not known, targets for therapy are based on main phenotypes of frataxin deficiency, including antioxidant defense (idebenone, CoQ10 plus vitamin E), improvement of respiration (idebenone, CoQ10 plus vitamin E), reduction of the mitochondrial iron pools (deferiprone), and increase in frataxin protein (erythropoietin EPO, pioglitazone). A major goal is the finding of chemical drugs that alleviate the Fe-S cluster deficiency. Recent strategies target the GAA-expansion triplet repeat (sticky DNA structure and heterochromatin) and intend to increase the FXN gene expression (HDAC inhibitors, polyamides). Other therapeutic strategies focus on replacement of the mutated gene by gene therapy.
FIG. 15.
Comprehensive model of frataxin function. Frataxin is an iron-binding protein implicated in the delivery of iron to Fe-S cluster assembly and heme synthesis and, by doing so, regulates mitochondrial respiration and cellular iron homeostasis. Impaired regulation of antioxidant defenses, decreased respiration, iron accumulation in the mitochondria, and possibly induction of ROS-producing enzymes causes ROS accumulation in the cell, which results in mutagenesis and oxidative stress. Frataxin is implicated, most likely, indirectly in the signaling of antioxidant defense systems and in the pathways that lead to survival or to death.
Similar articles
- Missense mutations linked to friedreich ataxia have different but synergistic effects on mitochondrial frataxin isoforms.
Li H, Gakh O, Smith DY 4th, Ranatunga WK, Isaya G. Li H, et al. J Biol Chem. 2013 Feb 8;288(6):4116-27. doi: 10.1074/jbc.M112.435263. Epub 2012 Dec 26. J Biol Chem. 2013. PMID: 23269675 Free PMC article. - Friedreich Ataxia: From the Eye of a Molecular Biologist.
Muthuswamy S, Agarwal S. Muthuswamy S, et al. Neurologist. 2015 Sep;20(3):51-5. doi: 10.1097/NRL.0000000000000054. Neurologist. 2015. PMID: 26375377 Review. - Exenatide induces frataxin expression and improves mitochondrial function in Friedreich ataxia.
Igoillo-Esteve M, Oliveira AF, Cosentino C, Fantuzzi F, Demarez C, Toivonen S, Hu A, Chintawar S, Lopes M, Pachera N, Cai Y, Abdulkarim B, Rai M, Marselli L, Marchetti P, Tariq M, Jonas JC, Boscolo M, Pandolfo M, Eizirik DL, Cnop M. Igoillo-Esteve M, et al. JCI Insight. 2020 Jan 30;5(2):e134221. doi: 10.1172/jci.insight.134221. JCI Insight. 2020. PMID: 31877117 Free PMC article. - Molecular Mechanisms and Therapeutics for the GAA·TTC Expansion Disease Friedreich Ataxia.
Gottesfeld JM. Gottesfeld JM. Neurotherapeutics. 2019 Oct;16(4):1032-1049. doi: 10.1007/s13311-019-00764-x. Neurotherapeutics. 2019. PMID: 31317428 Free PMC article. Review. - Neurodegeneration in Friedreich's ataxia: from defective frataxin to oxidative stress.
Gomes CM, Santos R. Gomes CM, et al. Oxid Med Cell Longev. 2013;2013:487534. doi: 10.1155/2013/487534. Epub 2013 Jul 9. Oxid Med Cell Longev. 2013. PMID: 23936609 Free PMC article. Review.
Cited by
- NMR as a Tool to Investigate the Processes of Mitochondrial and Cytosolic Iron-Sulfur Cluster Biosynthesis.
Cai K, Markley JL. Cai K, et al. Molecules. 2018 Aug 31;23(9):2213. doi: 10.3390/molecules23092213. Molecules. 2018. PMID: 30200358 Free PMC article. Review. - Co-precipitation of phosphate and iron limits mitochondrial phosphate availability in Saccharomyces cerevisiae lacking the yeast frataxin homologue (YFH1).
Seguin A, Santos R, Pain D, Dancis A, Camadro JM, Lesuisse E. Seguin A, et al. J Biol Chem. 2011 Feb 25;286(8):6071-9. doi: 10.1074/jbc.M110.163253. Epub 2010 Dec 28. J Biol Chem. 2011. PMID: 21189251 Free PMC article. - The transcriptional regulator CCCTC-binding factor limits oxidative stress in endothelial cells.
Roy AR, Ahmed A, DiStefano PV, Chi L, Khyzha N, Galjart N, Wilson MD, Fish JE, Delgado-Olguín P. Roy AR, et al. J Biol Chem. 2018 Jun 1;293(22):8449-8461. doi: 10.1074/jbc.M117.814699. Epub 2018 Apr 2. J Biol Chem. 2018. PMID: 29610276 Free PMC article. - Friedreich's Ataxia: A Neuronal Point of View on the Oxidative Stress Hypothesis.
Carletti B, Piemonte F. Carletti B, et al. Antioxidants (Basel). 2014 Sep 10;3(3):592-603. doi: 10.3390/antiox3030592. Antioxidants (Basel). 2014. PMID: 26785073 Free PMC article. Review. - Inducible and reversible phenotypes in a novel mouse model of Friedreich's Ataxia.
Chandran V, Gao K, Swarup V, Versano R, Dong H, Jordan MC, Geschwind DH. Chandran V, et al. Elife. 2017 Dec 19;6:e30054. doi: 10.7554/eLife.30054. Elife. 2017. PMID: 29257745 Free PMC article.
References
- Acquaviva F. Castaldo I. Filla A. Giacchetti M. Marmolino D. Monticelli A. Pinelli M. Saccà F. Cocozza S. Recombinant human erythropoietin increases frataxin protein expression without increasing mRNA expression. Cerebellum. 2008;7:360–365. - PubMed
- Acquaviva F. De Biase I. Nezi L. Ruggiero G. Tatangelo F. Pisano C. Monticelli A. Garbi C. Acquaviva AM. Cocozza S. Extra-mitochondrial localisation of frataxin and its association with IscU1 during enterocyte-like differentiation of the human colon adenocarcinoma cell line Caco-2. J Cell Sci. 2005;118:3917–3924. - PubMed
- Adinolfi S. Iannuzzi C. Prischi F. Pastore C. Iametti S. Martin SR. Bonomi F. Pastore A. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat Struct Mol Biol. 2009;16:390–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous