Seasonal hippocampal plasticity in food-storing birds - PubMed (original) (raw)
Review
Seasonal hippocampal plasticity in food-storing birds
David F Sherry et al. Philos Trans R Soc Lond B Biol Sci. 2010.
Abstract
Both food-storing behaviour and the hippocampus change annually in food-storing birds. Food storing increases substantially in autumn and winter in chickadees and tits, jays and nutcrackers and nuthatches. The total size of the chickadee hippocampus increases in autumn and winter as does the rate of hippocampal neurogenesis. The hippocampus is necessary for accurate cache retrieval in food-storing birds and is much larger in food-storing birds than in non-storing passerines. It therefore seems probable that seasonal change in caching and seasonal change in the hippocampus are causally related. The peak in recruitment of new neurons into the hippocampus occurs before birds have completed food storing and cache retrieval for the year and may therefore be associated with spacing caches, encoding the spatial locations of caches, or creating a neuronal architecture involved in the recollection of cache sites. The factors controlling hippocampal plasticity in food-storing birds are not well understood. Photoperiodic manipulations that produce change in food-storing behaviour have no effect on either hippocampal size or neuronal recruitment. Available evidence suggests that changes in hippocampal size and neurogenesis may be a consequence of the behavioural and cognitive involvement of the hippocampus in storing and retrieving food.
Figures
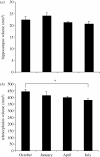
Figure 1.
(a) Hippocampus volume and (b) telencephalon volume in black-capped chickadees collected at four times during the year. Sample sizes are: October n = 6, January n = 5, April n = 6, July n = 7. There are no significant differences between months in hippocampal size or size of the hippocampus relative to the telencephalon. Asterisk indicates a significant difference between months (post hoc test p < 0.05). Error bars = 1 s.e.m. Adapted from Hoshooley et al. (2007).
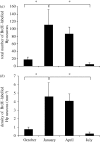
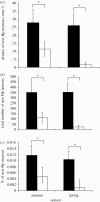
Figure 2.
(a) Total number of BrdU-labelled hippocampal (Hp) cells and (b) mean density of BrdU-labelled hippocampal cells in black-capped chickadees collected at four times of year. BrdU was administered on the day following capture and birds were sacrificed 6 days later. Sample sizes as for figure 1. Month of capture significantly affects both the total number of labelled cells and the density of labelled cells (_F_3,17 > 5.1, p < 0.01). Asterisks indicate significant differences between months (post hoc tests p < 0.05). Error bars = 1 s.e.m. Adapted from Hoshooley et al. (2007).
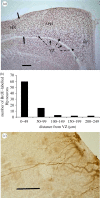
Figure 3.
(a) Black-capped chickadee hippocampus. The avian hippocampus consists of the hippocampus (HP) and the area parahippocampalis (APH). Solid arrows mark the boundary with hyperpallium apicale (HA). The ventricular zone (VZ), shown by dashed arrows, is the region of stem cell division that produces both neurons and radial glial cells. Cells are labelled for the neuronal nuclei specific protein NeuN. V, ventricle (scale bar, 200 µm). (b) Distances new neurons have travelled from the VZ six days following administration of the mitotic cell division marker BrdU, are shown in the bar graph. (c) Radial glial processes of cells labelled for the glial fibrillary acidic protein (GFAP). Glial cell bodies remain in the VZ while new neurons move along the radial processes into the hippocampus and other regions of the telencephalon (scale bar, 30 µm). (a) Adapted from Sherry & Hoshooley (2009). (b) Adapted from Hoshooley et al. (2007).
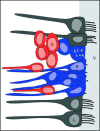
Figure 4.
The ventricular zone of adult birds showing ependymal cells (E) in grey, radial glial cells (B) in blue and new neurons (A) in red. Ependymal cells have multiple cilia extending into the lateral ventricle (V) while radial glia have only a single cilium. Radial glia divide to produce new neurons which migrate along the processes of radial glial cells (elongated cells shown in red) as well as moving parallel to the ventricle (oval cells shown in red). New neurons are not in contact with the ventricle. Glia nuclei may move close to the ventricle at the time of cell division. Adapted from Doetsch (2003) based on data from Alvarez-Buylla et al. (1998) and García-Verdugo et al. (2002).
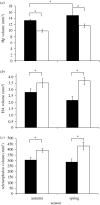
Figure 5.
More new neurons are found in the chickadee hippocampus (solid bar) than in the house sparrow hippocampus (open bar) in both spring and autumn. There was no difference between autumn and spring in hippocampal neurogenesis in chickadees in this study. (a) The density of new hippocampal neurons per mm3, (b) the number of new hippocampal neurons and (c) the per cent of all hippocampal neurons that are new. Birds received BrdU the day following capture and were sacrificed 6 weeks later. Sample sizes are: autumn chickadees, n = 9; autumn house sparrows, n = 5; spring chickadees, n = 6; spring house sparrows, n = 4. For differences between species on all measures, _F_1,20 > 6.3, p < 0.02. Asterisks indicate significant differences by post hoc tests p < 0.05. Error bars = ±1 s.e.m. Adapted from Hoshooley & Sherry (2007).
Figure 6.
(a) The black-capped chickadee hippocampus (solid bar) is larger than the house sparrow hippocampus (open bar) in both autumn and spring. (b) House sparrows are larger birds with larger brains and thus have a larger hyperpallium apicale (HA) and (c) telencephalon than chickadees. Chickadees, in this study, also had a larger hippocampus in spring than in autumn (a). Samples sizes as for figure 5. Differences betweens species for all three brain areas _F_1,20 > 12.4, p < 0.002. Asterisks indicate significant differences by post hoc tests, p < 0.05. Error bars = ±1 s.e.m. Adapted from Hoshooley & Sherry (2007).
Similar articles
- The seasonal hippocampus of food-storing birds.
Sherry DF, Hoshooley JS. Sherry DF, et al. Behav Processes. 2009 Mar;80(3):334-8. doi: 10.1016/j.beproc.2008.12.012. Behav Processes. 2009. PMID: 20522321 Review. - The hippocampal complex of food-storing birds.
Sherry DF, Vaccarino AL, Buckenham K, Herz RS. Sherry DF, et al. Brain Behav Evol. 1989;34(5):308-17. doi: 10.1159/000116516. Brain Behav Evol. 1989. PMID: 2611638 - Annual cycle of the black-capped chickadee: seasonality of food-storing and the hippocampus.
Hoshooley JS, Phillmore LS, Sherry DF, Macdougall-Shackleton SA. Hoshooley JS, et al. Brain Behav Evol. 2007;69(3):161-8. doi: 10.1159/000096984. Epub 2006 Nov 13. Brain Behav Evol. 2007. PMID: 17106193 - Behavioural and neural bases of orientation in food-storing birds.
Sherry D, Duff S. Sherry D, et al. J Exp Biol. 1996;199(Pt 1):165-72. doi: 10.1242/jeb.199.1.165. J Exp Biol. 1996. PMID: 9317563 - Seasonal change in the avian hippocampus.
Sherry DF, MacDougall-Shackleton SA. Sherry DF, et al. Front Neuroendocrinol. 2015 Apr;37:158-67. doi: 10.1016/j.yfrne.2014.11.008. Epub 2014 Dec 10. Front Neuroendocrinol. 2015. PMID: 25497862 Review.
Cited by
- Unified theory of Alzheimer's disease (UTAD): implications for prevention and curative therapy.
Nehls M. Nehls M. J Mol Psychiatry. 2016 Jul 15;4:3. doi: 10.1186/s40303-016-0018-8. eCollection 2016. J Mol Psychiatry. 2016. PMID: 27429752 Free PMC article. Review. - Seasonal Changes in Hematological Parameters in House Sparrows of Subtropical Pakistan.
Nimra S, Kayani AR, Irfan M, Ahmed MS. Nimra S, et al. Integr Org Biol. 2023 Jul 26;5(1):obad027. doi: 10.1093/iob/obad027. eCollection 2023. Integr Org Biol. 2023. PMID: 37549037 Free PMC article. - The effect of monocular occlusion on hippocampal c-Fos expression in domestic chicks (Gallus gallus).
Morandi-Raikova A, Mayer U. Morandi-Raikova A, et al. Sci Rep. 2020 Apr 29;10(1):7205. doi: 10.1038/s41598-020-64224-9. Sci Rep. 2020. PMID: 32350337 Free PMC article. - Possible linkage between neuronal recruitment and flight distance in migratory birds.
Barkan S, Roll U, Yom-Tov Y, Wassenaar LI, Barnea A. Barkan S, et al. Sci Rep. 2016 Feb 24;6:21983. doi: 10.1038/srep21983. Sci Rep. 2016. PMID: 26905978 Free PMC article. - Exploring the Role of Cognition in the Annual Fall Migration of the Monarch Butterfly (Danaus plexippus).
Gegear RJ. Gegear RJ. Insects. 2021 Aug 23;12(8):760. doi: 10.3390/insects12080760. Insects. 2021. PMID: 34442326 Free PMC article.
References
- Alvarez-Buylla A., Nottebohm F.1988Migration of young neurons in adult avian brain. Nature 335, 353–354 (doi:10.1038/335353a0) - DOI - PubMed
- Alvarez-Buylla A., Theelen M., Nottebohm F.1990Proliferation ‘hotspots’ in adult avian ventricular zone reveal radial cell division. Neuron 5, 101–109 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources